We explain what a heterogeneous mixture is, how it is made and various examples. Also, differences with a homogeneous mixture.

What is a heterogeneous mixture?
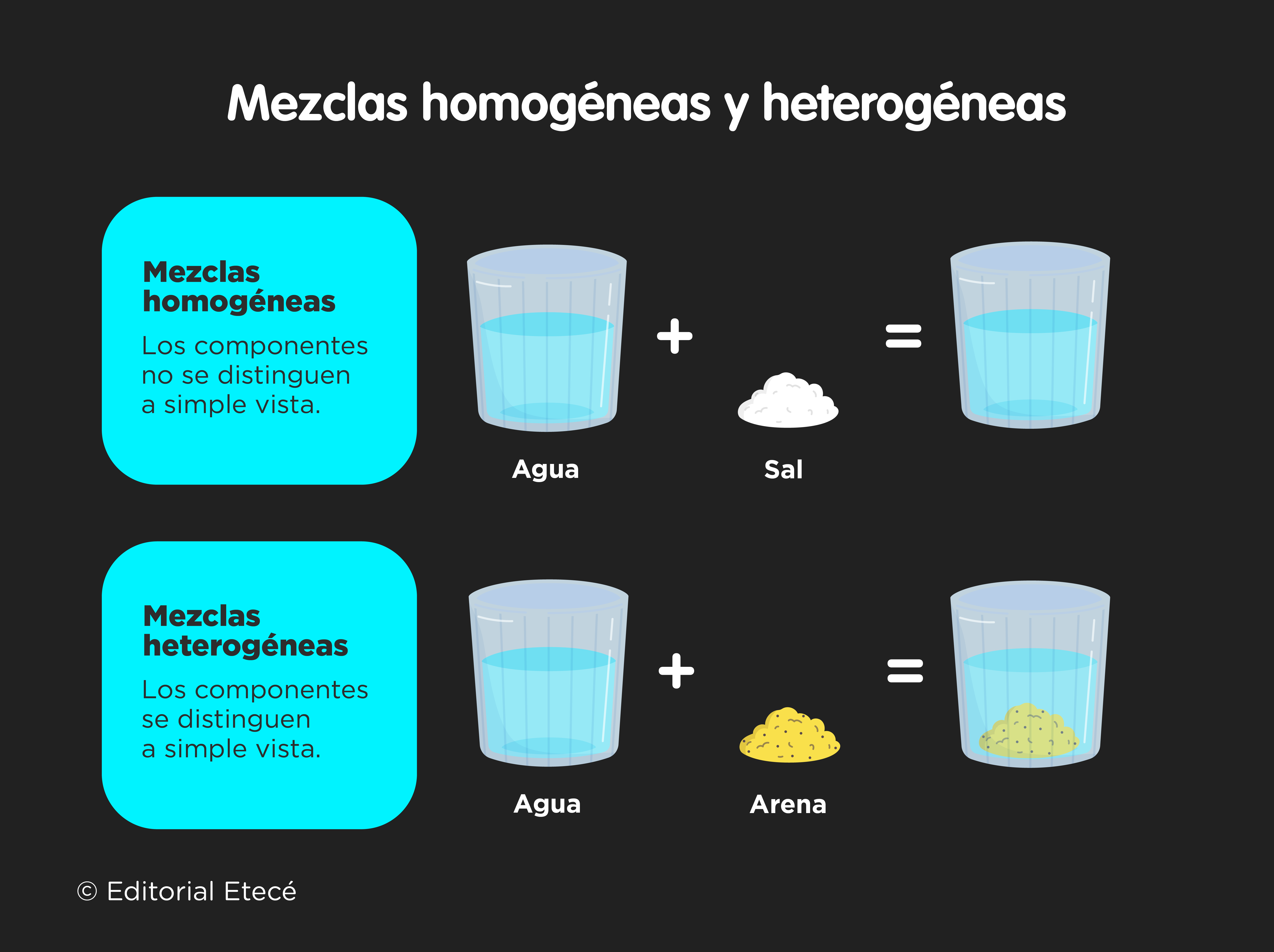
A heterogeneous mixture is a material composed by the union of two or more substances not chemically linked. Its fundamental characteristic is that its components are usually easily distinguished from each other. This type of mixture is not the product of a chemical reaction, although the mixture itself can then give rise to some type of reaction.
A heterogeneous mix It can be composed of solids, liquids, gases, or combinations between them. Generally these mixtures are produced by mechanical mixing procedures, during which no significant changes occur in the substances comprising the mixture.
However, although the substances retain their identities, the mixture can give rise to suspensions, colloids and other forms whose separation is not so simple.
To separate the components of a mixture there are mechanisms for separating mixtures which are usually physical procedures through which these components can be separated. Some examples are:
- Decantation
- Evaporation
- Filtration
- Sifting
- Centrifugation
- Magnetic separation
- Dissolution
Some of these mechanisms (such as filtration, sieving, centrifugation, and magnetic separation) can be used to separate heterogeneous mixtures with little effort. In the case of homogeneous mixtures, which are those mixtures in which the substances that make them up cannot be distinguished with the naked eye, more complex procedures must be used to separate the elements.
See also: Physical phenomena
Examples of heterogeneous mixtures

Some examples of heterogeneous mixtures are:
- The concrete. It is a mixture of cement, water and aggregates in specific proportions to form a paste that is used in construction.
- water with oil. It is a mixture formed by these two elements that, being immiscible, remain apart from each other (together but not mixed) forming clearly recognizable bubbles.
- The paste. It is a mixture formed by flour and water, in which the union of both materials can be clearly seen and, although they cannot be easily separated, the solid can be distinguished from the liquid with the naked eye.
- a salad. It is a heterogeneous mixture of various vegetables, seeds and other types of foods that are eaten together, but can be separated.
- air and gasoline. It is a mixture of fuel and air that is made inside an internal combustion engine that allows the controlled explosion of the fuel that generates movement.
| Oil and vinegar | shaving foam | Mayonnaise |
| Aerosol | Sodas | Pizza |
| Water with oil | Granite | salt and pepper |
| Water with sand | granola | Blood |
| Water with kerosene | Smoke | Smog |
| Broth | Oatmeal soap | Soda |
| Cereals with milk | Fruit juice with pulp | vegetable soup |
| Beer | Lava | Floor |
| nail polish | Milk | Indian ink |
Heterogeneous mixture and homogeneous mixture
The fundamental difference between a heterogeneous mixture and a homogeneous mixture is that in the first case the components can be identified more or less easily with the naked eye, while in the In homogeneous mixtures it is impossible to identify the components with the naked eye.
A homogeneous mixture does not allow us to distinguish its components, even though there has been no chemical reaction between them and they remain individual.
A clear example of a homogeneous mixture is the mixture of water and alcohol. Although both continue to retain their identities, it is impossible to distinguish one from the other and, therefore, their separation requires procedures that take into account the chemical nature of each, such as decantation or selective evaporation.
How to make a heterogeneous mixture?

Making a heterogeneous mixture is very simple, you simply have to mechanically combine two or more materials that can be recognized with the naked eye. Mechanical combination can be done by physically combining them, shaking them in a container or mixing them in the same container.
For example: If we take a paper hole punch and choose several sheets of paper of different colors we can pierce them several times each and accumulate a set of paper circles of different colors inside the device. If we think that each color represents a different component, we are in the presence of a heterogeneous mixture.
Continue with: Solute and solvent
References
- “Homogenous and heterogenous mixtures” in Lumen.
- “Example of heterogeneous mixture” (video) in Easy Experiments.
- “Mixtures and substances” (PDF) in ICT Resources.
- “Separation of heterogeneous mixtures” in Junta de Andalucía.
- “Heterogeneous and homogeneous mixtures” in EDUCANDO.





