Understand what the sulfur cycle is and what its stages consist of. In addition, we explain its characteristics and importance.
What is the sulfur cycle?
The biogeochemical sulfur cycle is the circulation of sulfur on planet Earth. Through this cycle, sulfur is transported, forming different chemical compounds between soil, water, the atmosphere and living organisms.
This cycle allows the exchange and use of sulfur by terrestrial and aquatic living beings who incorporate this element into their organisms in different ways. In this sense, plants incorporate sulfur present in the soil or water through their roots, while animals incorporate sulfur when they eat plants or when they eat other animals that previously ate plants. Thus, living beings can use sulfur to form their proteins and carry out their vital functions.
See also: Sulfur
Characteristics of the sulfur cycle
The sulfur cycle has some specific characteristics depending on how this element is transported and forms part of different chemical compounds, which in turn can exist in different states of aggregation and be absorbed by living beings.
Some of the characteristics of the sulfur cycle are:
- It guarantees that sulfur is part of different chemical compounds that are used by various living organisms.
- During the cycle, sulfur undergoes different changes of aggregation state (solid, liquid, gaseous).
- In this cycle, most of the sulfur is found in the lithosphere, forming part of fossil fuels.
- This cycle is involved in part of the formation of acid rain, which is harmful to plants, animals and buildings made by humans.
Stages of the sulfur cycle
During the sulfur cycle, this chemical element moves between different living organisms, and between living organisms and the environment that surrounds them.
This process occurs through a series of stages in which sulfur forms different chemical compounds and changes its state of aggregation (solid, liquid, gaseous). The sulfur cycle is made up of several stages.
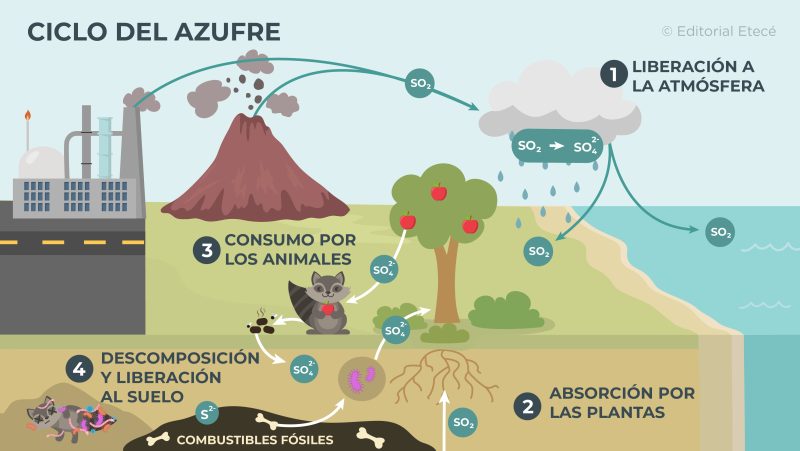
- Release to the atmosphere. Sulfur is expelled into the atmosphere due to the eruption of volcanoes and the industrial activity of man. It is released as part of sulfur dioxide (SO2), which is then transformed into sulfate ions (SO42-) and returns to the ground as part of acid rain.
- Sulfur absorption by plants Plants incorporate sulfur in the form of sulfate ions (SO42-) through its roots, and they use it to carry out the functions of their body.
- Consumption by animals Herbivorous animals (that feed mainly on plants) take in sulfur when they eat plants, while carnivorous animals (that eat other animals) take in sulfur when they eat other animals.
- Decomposition and release to the soil Some organisms, such as bacteria and fungi, have the ability to decompose dead organisms, and thus release the sulfur they contain in their bodies into the soil. In this way, the sulfur is absorbed again by the plants.
Importance of the sulfur cycle
The sulfur cycle is of vital importance since:
- Allows the recycling of sulfur throughout the planet in this way sulfur can be used by different living organisms and in the different industrial processes carried out by humans.
- Influences the formation of acid rain since sulfur dioxide (SO2) and sulfur trioxide (SO3) are part of the main components of acid rain, which causes serious damage to the environment.
- Ensures that sulfur can be part of some proteins and other molecules important for living organisms.
- Helps make sulfur available to be used in the different industries created by humans. For example:
➔ In the production of sulfuric acid (H2SW4), a fundamental compound in the economy of many countries (today the production of H2SW4 It is used to measure the economic development of certain countries).
➔ In the production of pharmaceutical products, fertilizers, cleaning products and in the oil refining process.
References
- Amengual, P., & Castellví, J. (1983). Sulfur cycle on the Mediterranean continental shelf.
- Mir, J. (1998). The biogeochemical cycle of sulfur in stratified ecosystems. Role of inorganic sulfur compounds (Doctoral dissertation, Universitat Autònoma de Barcelona).
- Colacelli, N. (2001). Sulfur in the ground. TECNIBOOK EDITIONS.