Learn what nitrogen is, its characteristics and properties. In addition, you will know where it is located, its importance, uses and history.

What is nitrogen?
Nitrogen is a chemical element of the Periodic Table that is represented with the symbol N. It has atomic number 7 and atomic mass 14,007 amu. It is a nonmetal and at normal pressure and temperature (1 atm and 20 °C) it exists as dinitrogen (N2), a molecular gas.
This chemical element is present in living beings since it is part of proteins, deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and adenosine triphosphate (ATP).
On the other hand, nitrogen constitutes 78% of the Earth's atmosphere, where it is present in the form of molecular nitrogen (N2), which is a very stable molecule, so it is difficult for it to react with other molecules to form chemical compounds. However, nitrogen undergoes some chemical reactions in the atmosphere.
In addition, this element is widely used to produce fertilizers and is part of chemical products widely used industrially, such as ammonia.


See also: Structure of DNA
Nitrogen properties
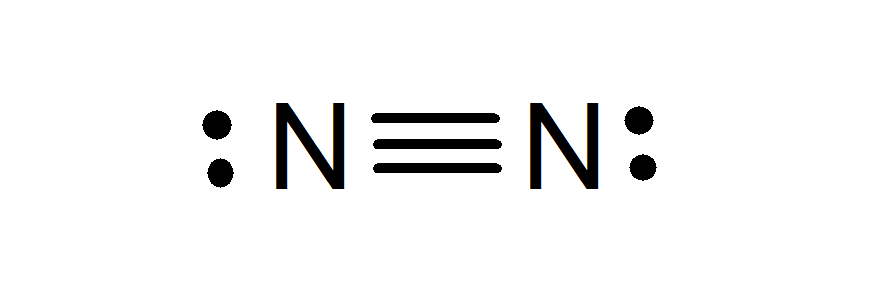
Nitrogen in its molecular form (N2) hardly undergoes chemical reactions because it is very stable. However, it undergoes some chemical reactions, especially those related to the formation of ammonia (NH3), a chemical compound widely used in industry.
Physical properties of nitrogen
Some physical properties of nitrogen are:
- It is a colorless, odorless and tasteless gas under normal conditions of pressure and temperature.
- It is a non-metal.
- It is not a good conductor of heat and electricity.
- Its melting point is -210 °C.
- Its boiling point is -196 °C.
Chemical properties of nitrogen
Some chemical properties of nitrogen are:
- It has primary oxidation state -3.
- Reacts with lithium in the presence of heat to form lithium nitride (Li3N), joins a chemical compound that is the only stable nitride formed with an alkali metal (metals of Group I of the Periodic Table (lithium, sodium, potassium, rubidium, cesium and francium)).
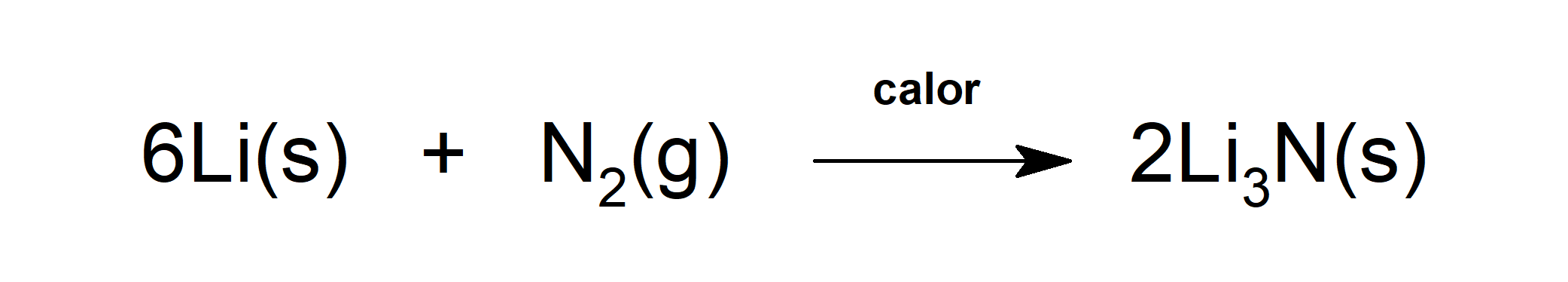
- Reacts with dihydrogen (H2) to form ammonia (NH3).
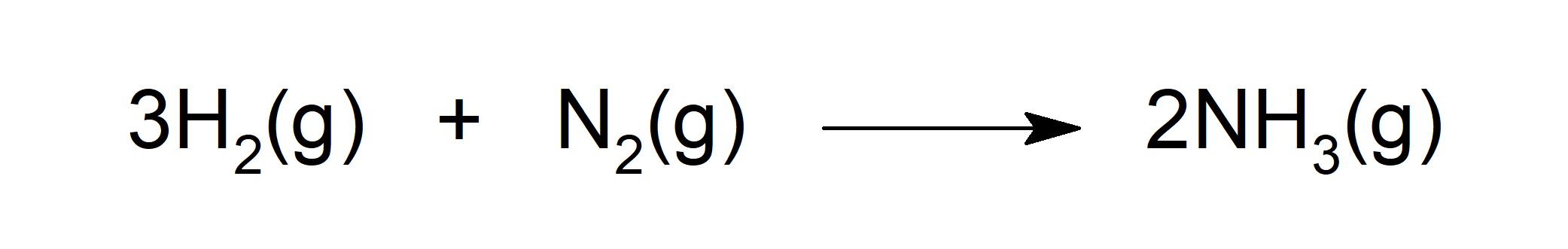
- Reacts with oxygen (O2) to form different oxides such as nitrogen monoxide (NO). NO, in turn, reacts with O2 to form nitrogen dioxide (NO2).
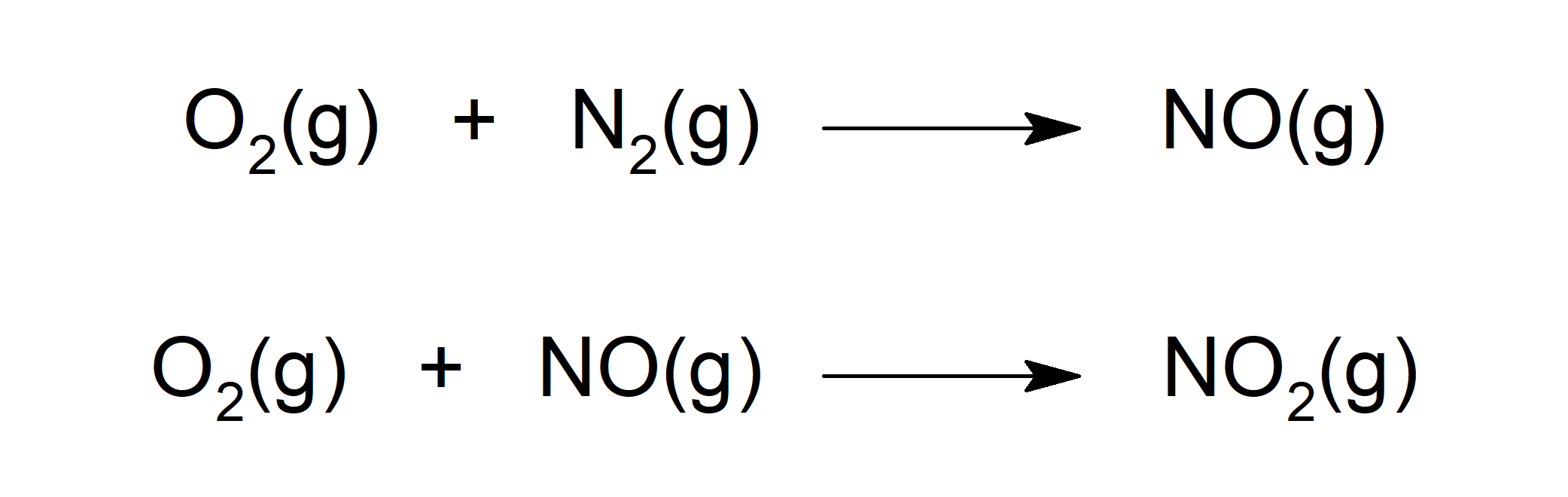
What is nitrogen used for?

Nitrogen has various applications in different processes carried out by people. One of its main uses is as a starting component in the production of ammonia (N.H.3). In addition, it is used to inflate airplane tires, in the production of stainless steel, in extinguishing fires and in the manufacture of light bulbs.
Besides, There are many chemical compounds that contain nitrogen or that are widely used by man in different activities, such as:
- Potassium nitrate (KNO3) is used in the production of gunpowder.
- Ammonia (NH3) and ammonium nitrate (NH4NO3) are used in the production of fertilizers.
- Nitroglycerin (C3h5N3EITHER9) and trinitrotoluene (C7h5N3EITHER6) are used as explosives.
- Hydrazine (N2h4) has been used as part of rocket fuels.
Where is nitrogen found?
Nitrogen makes up 78% of the atmosphere. Furthermore, this chemical element is part of living beings since it is present in proteins: in the human body it occupies 3% of the composition of chemical elements.
On the other hand, nitrogen is present in waste products of living beings such as guano, urea and uric acid. It is also found in the form of nitrate ions (NO3–), on the Earth's surface and in the planet's waters.
Biological importance of nitrogen
nitrogen It is a fundamental chemical element of the structure of amino acids which are the molecules that form proteins and constitute a fundamental part of living organisms, which is why they influence the formation and maintenance of organs and tissues.
Additionally, this element participates in the formation of hormones, antibodies and enzymes, It is part of DNA and RNA and plays a very important role in the functioning of muscle relaxation, the central and peripheral nervous system. This chemical element too.
Environmental effects of nitrogen
Nitrogen has been widely used in the production of fertilizers which when spread on crops, have caused an increase in this chemical element in the soils and waters of the planet.
This excess of nitrogen in soils and waters has caused the process of eutrophication: an excessive increase in algae and microorganisms when they have an excess of some nutrient available, such as nitrogen. The overpopulation of these algae and microorganisms causes a lot of dissolved oxygen to be consumed, which causes the death of various aquatic organisms.
On the other hand, nitrogen oxides contribute to the formation of acid rain, and specifically nitrous oxide (N2O) it is a powerful greenhouse gas. In addition, nitrogen-containing chemical compounds formed during combustion contribute to air pollution.
Nitrogen cycle
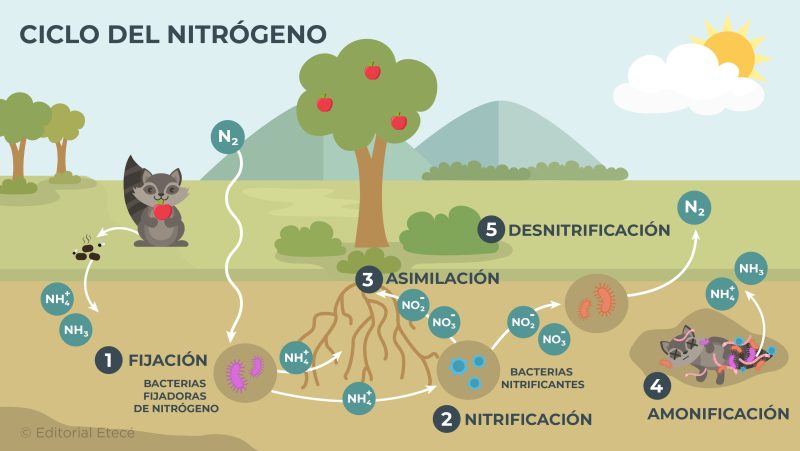
The nitrogen cycle is the circulation of nitrogen on planet Earth. Through this cycle, nitrogen moves between different ecosystems and the environment that surrounds them. This cycle is made up of different stages:
- Fixation It is the process by which organisms use nitrogen to carry out the chemical reactions necessary for their bodies to function correctly. Living beings use chemical compounds that contain nitrogen and were formed by the action of microorganisms or by the oxidation of atmospheric nitrogen (N2).
- Nitrification It is the process by which ammonia (NH3) or the ammonium ion (NH4+) are transformed into nitrite ions (NO2–) and nitrates (NO3–) by the action of specific microorganisms. These ions can be incorporated into plants and animals.
- Assimilation It is the process by which plants and animals incorporate nitrogen into their organisms. When these organisms die and decompose, nitrogen again returns to the surrounding environment.
- Ammonification It is the process by which the nitrogen present in living beings is released into the environment in the form of ammonia (NH3) or ammonium ion (NH4+). It occurs during the decomposition of dead organisms or during the excretion of waste (urine and feces) by living organisms.
- Denitrification It is the process by which microorganisms, such as denitrifying bacteria, break down nitrate ions (NO3–) and nitrite (NO2–) into nitrogen gas (N2), which is released into the atmosphere.
History of nitrogen
Some nitrogen-containing compounds have been known since ancient times. For example, alchemists knew about nitric acid (HNO3), which they called aqua fortis (strong water), and aqua regia (mixture of nitric acid (HNO3) and hydrochloric acid (HCl)), which was very important because it dissolves gold.
However, it is considered that Nitrogen was discovered in 1772 by the Scottish physician Daniel Rutherford who named him Harmful Air. Rutherford distinguished nitrogen from carbon dioxide (CO2), and although he had not identified nitrogen as a chemical element, he did observe that it was a component of air that did not undergo combustion reactions.
During those years, nitrogen was also studied by the Swedish chemist Carl Wilhelm Scheele, the French physicist and chemist Henry Cavendish and the British scientist Joseph Priestley, who named this element after burned air.
On the other hand, the French chemist Antoine Lavoisier called nitrogen “mephitic air” or “azote”, which means lifelessbecause this element is very inert and hardly undergoes chemical reactions. In 1790, the French chemist Jean-Antoine Chaptal introduced the word nitrogen from which the English word was derived in 1794 nitrogen.
The first applications of nitrogen were made using sodium nitrate (NaNO3) and potassium nitrate (KNO3) in industrial, agricultural and military applications, such as gunpowder production.
References
- Mayz-Figueroa, J. (2004). Biological nitrogen fixation. UDO Agricultural Scientific Magazine, 4(1), 1-20.
- Cárdenas-Navarro, R., Sánchez-Yáñez, JM, Farías-Rodríguez, R., & Peña-Cabriales, JJ (2004). Nitrogen contributions in agriculture. Chapingo Magazine Horticulture Series, 10(2), 173-178.
- Cerón Rincón, LE, & Ancízar Aristizábal Gutiérrez, F. (2012). Dynamics of the nitrogen and phosphorus cycle in soils. Colombian Journal of Biotechnology, 14(1), 285-295.
