We explain what the groups of the periodic table are and the characteristics of each one. Also, the periods of the periodic table.
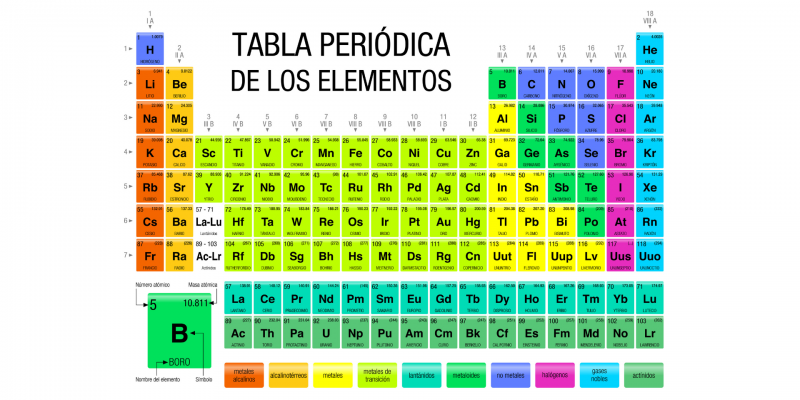
What are the groups of the periodic table?
In chemistry, the groups of the Periodic Table are the columns of elements that compose it corresponding to families of chemical elements that share many of their atomic characteristics.
In fact, the primary function of the Periodic Table, created by the Russian chemist Dmitri Mendeleyev (1834-1907), is precisely to serve as a classification and organization diagram of the different families of known chemical elements, so that the groups They are one of its most important components.
These groups are represented in the columns of the table, while the rows constitute the periods. There are 18 different groups, numbered from 1 to 18, each of which groups together a variable number of chemical elements. The elements in each group have the same number of electrons in their last atomic shell which is why they have similar chemical properties, because the chemical properties of the chemical elements are strongly related to the electrons located in the last atomic layer.
The numbering of the different groups within the table is currently established by the International Union of Pure and Applied Chemistry (IUPAC) and corresponds to the Arabic numbers (1, 2, 3… 18), in replacement of the traditional European method that used Roman numerals and letters (IA, IIA, IIIA… VIIIA) and the American method that also used Roman numerals and letters, but in another arrangement different from the European method.
- IUPAC. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18.
- European system IA, IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, VIIIA, VIIIA, IB, IIB, IIIB, IVB, VB, VIB, VIIB, VIIIB.
- American system IA, IIA, IIIB, IVB, VB, VIB, VIIB, VIIIB, VIIIB, VIIIB, IB, IIB, IIIA, IVA, VA, VIA, VIIA, VIIIA.
Thus, Each element present in the periodic table always corresponds to a specific group and period which reflect the way of classifying matter that humanity has developed scientifically.
See also: Atomic number
What are the groups of the periodic table?
Next, we will describe each of the groups of the Periodic Table using the IUPAC numbering and the old European system:
- Group 1 (formerly IA) or alkali metals Composed of the elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr), all common in plant ashes and basic in nature when they are part of oxides. They have low density, their own color and are usually soft. Hydrogen (H) is also usually included in this group, although it is also common for an autonomous position to be present among the chemical elements. Alkali metals are extremely reactive and need to be stored in oil to prevent them from reacting with moisture in the air. Furthermore, they are never found as free elements, that is, they are always part of some chemical compound.
- Group 2 (formerly IIA) or alkaline earth metals Composed of the elements beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra). The name “alkaline earth” comes from the name that its oxides received in ancient times (land). They are soft metals (although harder than those of group 1), of low density, good conductors and with electronegativity less than or equal to 1.57 according to the Pauling scale (scale established to organize the electronegativity values of atoms, where the fluorine (F) is the most electronegative and francium (Fr) is the least electronegative). They are less reactive elements than those of group 1, but even so, they are still very reactive. The last one on the list (Ra) is radioactive and has a very short half-life (time it takes for a radioactive atom to decay), so it is often not included in lists.
- Group 3 (formerly IIIA) or scandium family Composed of the elements scandium (Sc), yttrium (Y), lanthanum (La) and actinium (Ac), or lutetium (Lu) and laurentium (Lr) (there is debate among specialists about which of these elements should be included in this cluster). They are solid and shiny elements, very reactive and with a great tendency to oxidation, good for conducting electricity.
- Group 4 (formerly VAT) or titanium family Composed of the elements titanium (Ti), zirconium (Zr), hafnium (Hf) and rutherfordium (Rf), which are very reactive metals and that, when exposed to air, acquire a red color and can ignite spontaneously (that is, they are pyrophoric). The last (Rf) of the family is a synthetic and radioactive element.
- Group 5 (formerly VA) or vanadium family Composed of the elements vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db), metals that have 5 electrons in their outermost atomic layers. Vanadium is quite reactive since it has variable valence but the others are to a very small extent, and the last one (Db) is a synthetic element that does not exist in nature.
- Group 6 (formerly VIA) or chromium family Composed of the elements chromium (Cr), molybdenum (Mo), tungsten (W) and seaborgium (Sg), all transition metals, and Cr, Mo and W are refractory. They do not present uniform electronic characteristics, despite their similar chemical behavior.
- Group 7 (formerly VIIA) or manganese family Composed of the elements manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh), of which the first (Mn) is very common and the others extremely rare, especially technetium (which has no isotopes). stable) and rhenium (which exists only in traces in nature).
- Group 8 (formerly VIIIA) or iron family Composed of the elements iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs), transition metals that have eight electrons in their outer shells. The last one on the list (Hs) is a synthetic element that exists only in the laboratory.
- Group 9 (formerly VIIIA) or cobalt family Composed of the elements cobalt (Co), rhodium (Rh), iridium (Ir) and meitnerium (Mr), they are solid transition metals at room temperature, of which the last one (Mr) is synthetic and exists only in laboratories.
- Group 10 (formerly VIIIA) or nickel family Composed of the elements nickel (Ni), palladium (Pd), platinum (Pt) and darmstadtium (Ds), they are solid transition metals at room temperature, which are abundant in nature in their elemental form, except nickel, which has a enormous reactivity, which is why it exists forming chemical compounds, and is also abundant in meteorites. They have catalytic properties that make them very important in the chemical industry and aerospace engineering.
- Group 11 (formerly IB) or copper family Composed of the elements copper (Cu), silver (Ag), gold (Au) and roentgenium (Rg), called “coin metals” for their use as an input for coins and jewelry. Gold and silver are precious metals, copper on the other hand is very useful industrially. The only exception is roentgenium, which is synthetic and does not exist in nature. They are good electrical conductors, and silver has very high levels of heat conduction and light reflectance. They are very soft and ductile metals, widely used by humanity.
- Group 12 (formerly IIB) or zinc family Composed of the elements zinc (Zn), cadmium (Cd) and mercury (Hg), although different experiments with the synthetic element copernicium (Cn) could include it in the group. The first three (Zn, Cd, Hg) are abundantly present in nature, and the first two (Zn, Cd) are solid metals, with mercury being the only liquid metal at room temperature. Zinc is an important element for the metabolism of living beings, while the others are highly toxic.
- Group 13 (formerly IIIB) or boron family Composed of the elements boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh), they are also called “earth” since they are very abundant in the Earth's crust. , except for the last one on the list, synthetic and non-existent in nature. The industrial popularity of aluminum has led to the group also being known as the “aluminum group.” These elements have three electrons in their outer shell, they are metals with very low melting points, except boron, which has a very high melting point and is a metalloid.
- Group 14 (formerly IVB) or carbonoids Composed of the elements carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb) and flerovium (Fl), they are mostly well-known and abundant elements, especially carbon, central for the chemistry of living beings. This element is non-metallic, but as you go down the group the elements become more and more metallic, until you reach lead. They are also elements widely used in industry and very abundant in the Earth's crust (silicon constitutes 28% of it) except flerovium, synthetic and radioactive with a very short half-life.
- Group 15 (formerly VB) or nitrogenoids Composed of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi) and the synthetic element Moscovium (Mc), they are also known as pnicogens, they are very abundant and very reactive when at high temperatures. They have five electrons in their outer shell, and as in the previous group, they acquire metallic properties as we advance in the group.
- Group 16 (formerly VIB) or chalcogens or amphigens Composed of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po) and livermorium (Lv), they are, with the exception of the last one (Lv, synthetic), very common elements and used industrially. , the first two (O, S) also involved in the typical processes of biochemistry. They have six electrons in their outer atomic shell and some of them tend to form acidic or basic compounds, hence their name amphigenes (from the Greek amphi“on both sides”, and genos“produce”). Among the group, oxygen stands out, with a very small size and enormous reactivity.
- Group 17 (formerly VIIB) or halogens Composed of the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At) and tenese (Ts), they are usually found in their natural state as diatomic molecules that tend to form mononegative ions called halides. The last one on the list (the Ts), however, is synthetic and does not exist in nature. These are abundant elements in biochemistry, with enormous oxidation power (especially fluorine). Its name comes from the Greek words halos (“salt”) and genos (“produce”), that is, “salt producers”.
- Group 18 (formerly VIIIB) or noble gases Composed of the elements helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and oganeson (Og), its name comes from the fact that in nature they usually be in gaseous form and have a very low reactivity, which makes them excellent insulators for different industries. They have very close melting and boiling points, so they can be liquid only in a small range of temperatures, and with the exception of radon (very radioactive) and oganeson (synthetic), they are abundant in terrestrial air and in the universe. (especially helium, produced in the hearts of stars by hydrogen fusion).
Periods of the periodic table
Just as there are groups, represented in the form of columns, there are also periods that are horizontal rows of the periodic table. The periods are directly related to the energy levels of each element, that is, to the number of electronic orbits that surround the nucleus.
For example, iron (Fe) is in the fourth period that is, the fourth row of the table, since it has four electronic layers; while barium (Ba), having six layers, is in the sixth period, that is, the sixth row of the periodic table.
Continue with: Chemical formula
References
- “Periodic table of the elements” on Wikipedia.
- “Group of the periodic table” in Enciclopedia.us.
- “Groups of the periodic table” (video) on Khan Academy.
- “Groups and periods” in ICT Resources of the Spanish Ministry of Education.
- “Group (Periodic Table)” in The Encyclopaedia Britannica.





