We explain what the octet rule is in chemistry, who its creator was, examples and exceptions. Also, the Lewis structure.
What is the octet rule?
In chemistry, it is known as the octet rule or octet theory. the explanation of the way in which the atoms of chemical elements combine.
This theory was stated in 1917 by the American physicist and chemist Gilbert N. Lewis (1875-1946) and explains that the atoms of different elements usually always maintain a stable electronic configuration by placing eight electrons in their final energy levels.
The octet rule states that the ions of the different chemical elements found in the Periodic Table They usually complete their last energy levels with 8 electrons. Due to this, the molecules can acquire a stability similar to that of the noble gases (located at the far right of the periodic table), whose electronic structure (with its last complete energy level) makes them very stable, that is, not very reactive.
Thus, elements with high electronegativity (such as halogens and amphigens, that is, elements of group 16 of the Table) tend to “gain” electrons until they reach the octet, while those with low electronegativity (such as alkalines or alkaline earths) tend to to “lose” electrons to reach the octet.
This rule explains one of the ways atoms form their bonds and the behavior and chemical properties of the resulting molecules will depend on the nature of these. In this way, the octet rule is a practical principle that serves to predict the behavior of many substances, although it also presents various exceptions.
See also: Covalent bond
Examples of the octet rule
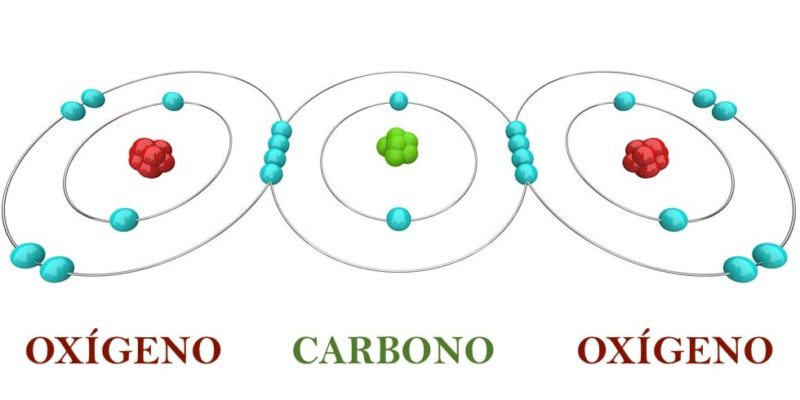
Let's think about a molecule of CO2 whose atoms have valences of 4 (carbon) and 2 (oxygen), linked by double chemical bonds. (It is important to clarify that valence are the electrons that a chemical element must give up or accept to ensure that its last energy level is complete. Chemical valence should not be confused with valence electrons, since the latter are the electrons that are are located in the last energy level).
This molecule It is stable if each atom has 8 electrons in total in its last energy level reaching the stable octet, which is fulfilled with the sharing of 2 electrons between carbon and oxygen atoms:
- Carbon shares two electrons with each oxygen, increasing the electrons in the last energy level of each oxygen from 6 to 8.
- At the same time, each oxygen shares two electrons with carbon, increasing from 4 to 8 electrons in the last energy level of carbon.
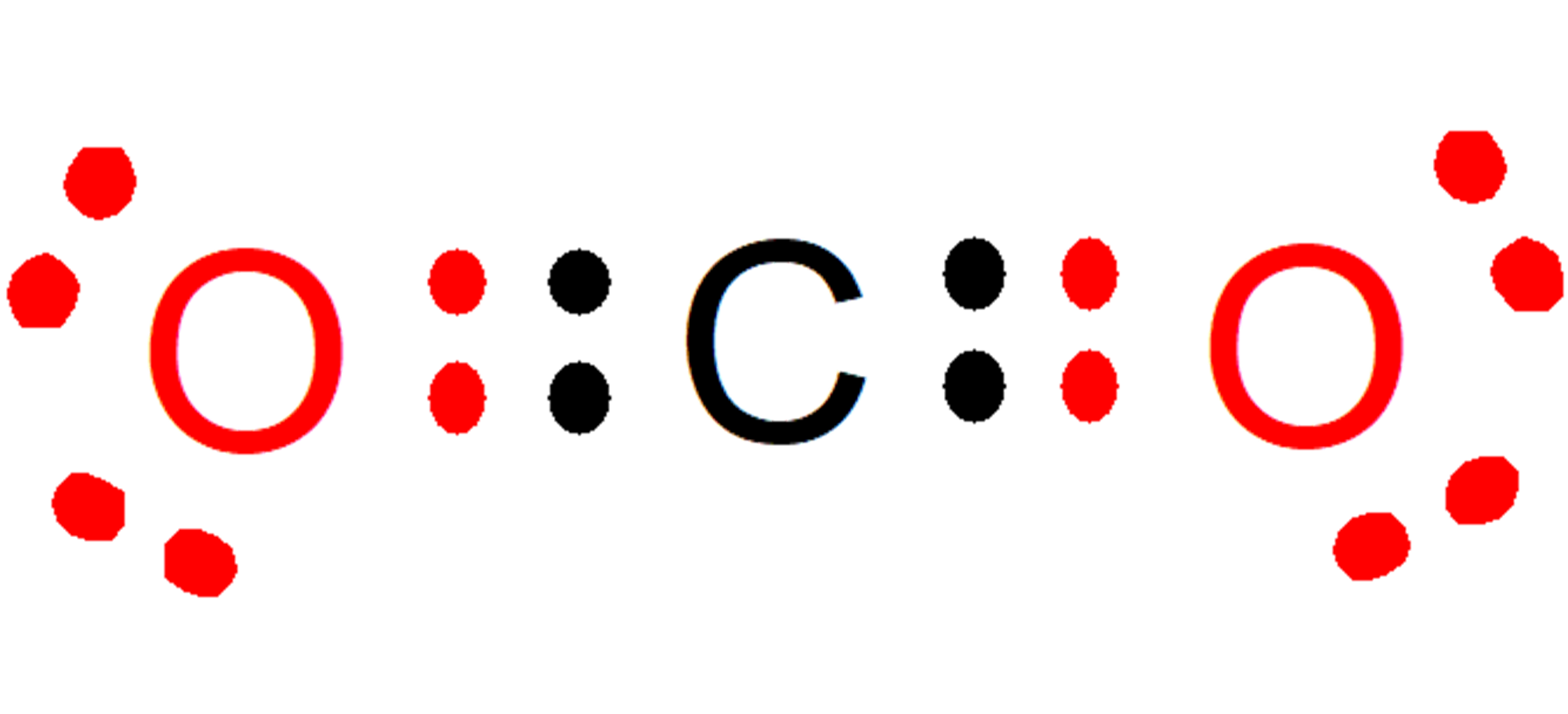
Another way of looking at it would be that The total of electrons given and taken must always be eight.
That is the case with other stable molecules, such as sodium chloride (NaCl). Sodium contributes its only electron (valence 1) to chlorine (valence 7) to complete the octet. Thus, we would have Na1+CL1- (that is, sodium gave up an electron, and gained a positive charge, and chlorine accepted an electron and with it a negative charge).
Exceptions to the octet rule
The octet rule has several exceptions, that is, compounds that reach their stability without being governed by the octet of electrons. Atoms like phosphorus (P), sulfur (S), selenium (Se), silicon (Si) or helium (He) they can accommodate more electrons than suggested by Lewis (hypervalence).
On the contrary, the hydrogen (H) which has a single electron in a single atomic orbital (region of space where an electron is most likely to be found around the atomic nucleus), can accept up to two electrons at most in a chemical bond. Other exceptions are the beryllium (Be) which acquires stability with just four electrons, or the boron (B) which does it with six.
Octet rule and Lewis structure
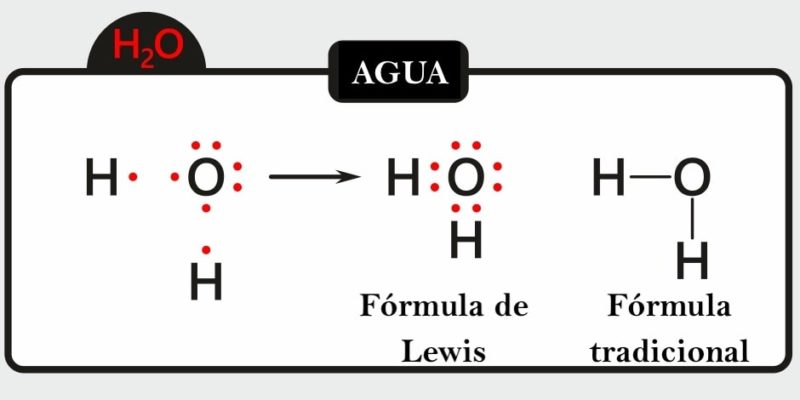
Another of Lewis's great contributions to chemistry was his famous way of representing atomic unions today known as the “Lewis structure” or “Lewis formula”.
It consists of placing dots or dashes to represent shared electrons in a molecule and the electrons that remain free on each atom.
This type of two-dimensional graphic representation allows us to know the valence of an atom that interacts with others in a compound and whether it forms single, double or triple bonds, all of which will affect the molecular geometry.
To represent a molecule in this way we need to choose a central atom which will be surrounded by the others (called terminals) establishing links until the valences of all those involved are reached. The former tend to be the least electronegative and the latter the most electronegative.
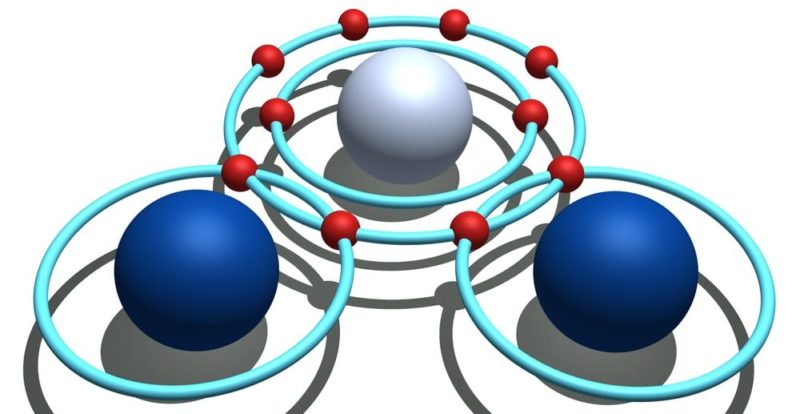
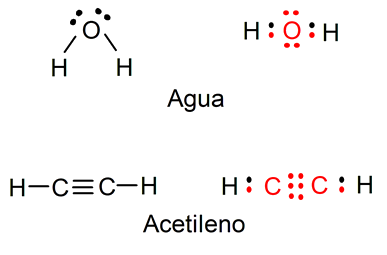
For example, the representation of water (H2O) shows the free electrons that the oxygen atom has you can also visualize the simple bonds between the oxygen atom and the hydrogen atoms (the electrons that belong to the oxygen atom are represented in red and those of the hydrogen atoms in black). Also represented is the acetylene molecule (C2h2), where you can visualize the triple bond between the two carbon atoms and the single bonds between each carbon atom and a hydrogen atom (the electrons that belong to the carbon atoms are represented in red and those of the carbon atoms hydrogen in black).

Continue with: Oxidation
References
- “Octet rule” https://es.wikipedia.org/
- “Octet Rule” (video) Oakademia – Study online and whenever you want. https://www.youtube.com/
- “Octet Rule” https://quimica.laguia2000.com/
- “What is the octet rule?” https://www.tplaboratorioquimico.com/