We explain what solubility is in chemistry and what factors affect it. Also, what is the solubility product and various examples.

What is solubility?
In chemistry, solubility is the capacity of a body or a given substance (called solute) to dissolve in a certain medium (called solvent); that is, it is the maximum amount of a solute that a solvent can receive under certain environmental conditions.
The solute is the substance that dissolves in a certain solvent. It can be a solid, a liquid or a gas. Generally, the solute is found in smaller quantities than the solvent in a solution.
The solvent or solvent is the substance in which a certain solute is dissolved. Generally, the solvent is found in greater quantity than the solute in a solution.
The solubility can be expressed by concentration units such as molarity or molality, for example.
The molar concentration (referred to molarity) is defined as the number of moles of solute per liter of solution (or equivalent unit), and is calculated as follows:


Where:
- M(X). Molarity of the substance x expressed in mol/L.
- n(X). Amount of substance of the substance x expressed in moles (mol).
- V(X). Volume of solution expressed in liters (L) or equivalent units.
The molal concentration (referred to molality) is defined as the number of moles of solute in one kilogram of solvent, and is calculated as follows:

Where:
- m(X). It is the molality of the substance x expressed in mol/(kg of solvent).
- n(X). It is the amount of substance of the substance x expressed in moles (mol).
- m(solvent expressed in kg). It is the mass of solvent expressed in kg.
However, solubility is not a universal characteristic of all substances. Some dissolve easily, others more difficult, and some simply do not dissolve.
It all also depends on what substances we are mixing. Water, commonly referred to as the universal solvent, cannot completely dissolve oil, for example.
But even when a solvent manages to dissolve a solute, it does so to a certain extent, which is why solutions can be classified as:
- Saturated When no more solute can be dissolved, that is, when the solution has the maximum solute that the solvent admits.
- Unsaturated. When more solute can continue to be dissolved in the solution.
- Oversaturated. When the solution has more solute than it can dissolve. A supersaturated solution can be achieved by modifying certain conditions, such as temperature, to dissolve more solute than the maximum that the solution supports.
See also: Solute and solvent
Factors affecting solubility

In principle, the solubility of a substance depends on which other substance we are mixing it with. Broadly speaking, substances are classified as:
- Water soluble They are those that can dissolve more easily (or completely) in water.
- Fat-soluble They are those that can dissolve more easily in oils.
On the other hand, the solubility of substances depends on the following factors:
Temperature. Most solids increase their solubility in water with increasing temperature, although there are some exceptions. Organic compounds also generally increase their solubility with increasing temperature. This increase in solubility with increasing temperature is due to the fact that the interactions between the particles of the solute and the solvent increase, so the intermolecular forces between them can be broken. On the other hand, gaseous solutes have a different behavior, since as the temperature increases, their solubility in organic solvents increases, but decreases in water because the gas tends to escape from the liquid with the increase in temperature.
For example, a glass of water dissolves a certain amount of sugar, until the excess begins to precipitate to the bottom. If we heat said glass of water, we will notice how the excess begins to disappear, increasing the solubility of the solute in the solvent.
Pressure. Pressure mainly influences the solubility of gaseous solutes. Increasing the pressure of a gaseous solute increases its solubility in a certain solvent.
Nature of the solute and the solvent Substances with the same polarity are soluble in each other, from which comes the phrase: “like dissolves like.” However, when a solute and a solvent have different polarities, they are completely insoluble in each other, although there is always a range of intermediate polarities in which a solute and a solvent can be partially soluble.
Polarity is a property of chemical compounds that have the tendency to separate electrical charges in their structure.
Polar molecules are made up of atoms whose electronegativity is very different, while nonpolar molecules are made up of atoms with the same electronegativity.
But the polarity of a molecule is also determined by the symmetry of its structure, so there may be molecules formed by atoms whose electronegativity is different, but they are arranged in such a way in the molecular structure that their dipoles cancel out and finally the molecule It is nonpolar.
Agitation. Shaking or stirring the solutions increases the solubility of the solute, as it contributes to greater interaction between the solute and the solvent.
See also: Van der Waals forces
Solubility product
When we talk about solubility product or ionic product (abbreviated Konly Ks), we refer to the product of the molar concentrations of the ions that form a compound raised to their respective stoichiometric indices of the equilibrium equation. Thus, the greater the Ksun, the more soluble the compound will be. This is expressed with the following formula, considering the equilibrium equation:
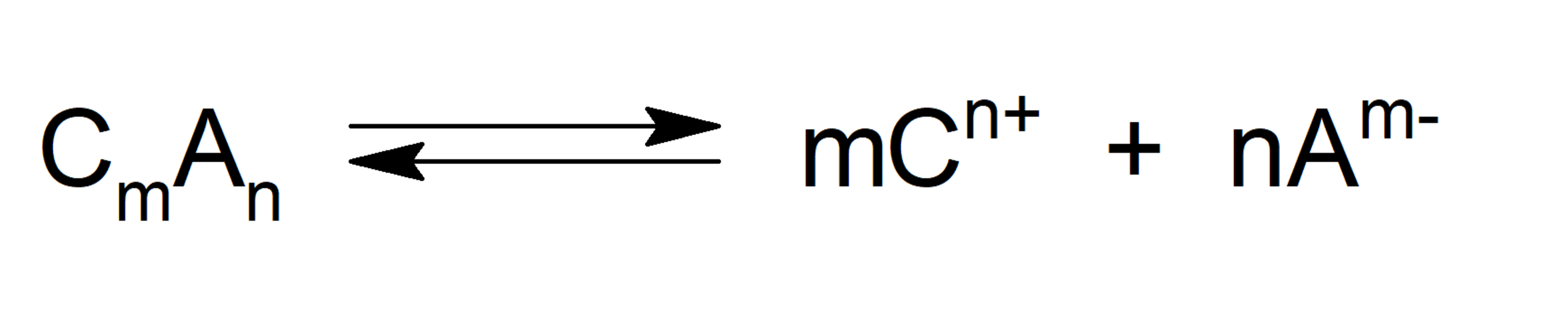
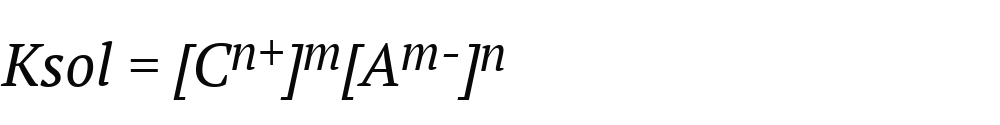
Where:
- Ksol. It is the product of solubility.
- (Cn+)m It is the molar concentration of the cation raised to the stoichiometric coefficient m.
- (TOm-)n It is the molar concentration of the anion raised to the stoichiometric coefficient. n.
Examples of solubility

- Salt dissolved in water Common salt (sodium chloride, NaCl) dissolves easily in water, at a rate of 360 grams per liter, as long as the water is at 20 ºC. If we increase the temperature of the solvent, the amount of salt we can dissolve will increase.
- Fizzy drinks The canned or bottled soft drinks that we consume daily have an amount of carbon dioxide (CO2) gas dissolved inside, and that is why they have their characteristic bubbling. To achieve this, industries supersaturate the mixture at very high pressure conditions. Therefore, when we uncover them, the pressure balances and a gas leak begins.
- Iodized solutions We often use iodine solutions to heal superficial wounds, which cannot be made with water, since iodine is not soluble in water. That is why they use alcohol, whose solubility rate improves and allows the mixture to be produced.
- Coffee with milk To prepare a coffee with milk, we add the milk to the infusion and observe how it changes in color as it mixes. This is always done with hot coffee, since the solubility rate of both substances increases with temperature. If we wait for the substances to cool, however, we will notice the formation of cream on the surface, since the solution has become saturated more quickly.
Continue with: Chemical solution
References
- “Solubility” https://es.wikipedia.org/
- “Solubility” (video) in Educatina. https://www.youtube.com/
- “Solubility” (video) in Profe en c@sa. https://www.youtube.com/
- “Solubility” https://www.portaleducativo.net/
- “Solubility – an Overview” https://www.sciencedirect.com/
- “Solubility” https://www.britannica.com/





