We explain what metalloids are, what their uses and characteristics are. Also, the use of the term “semimetals.”

What are metalloids?
Metalloids or semimetals are certain types of chemical elements that exhibit intermediate behavior between metallic and non-metallic elements, with regard to matters of ionization and bonding properties. They are elements that act like metals in some situations and like non-metals in others.
However, it is not easy to distinguish metalloids from true metals, and doing so generally requires a review of their electrical conduction properties, since they also tend to vary greatly from one another in shape, appearance, and coloration.
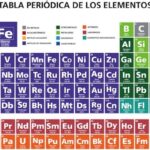
The elements known as metalloids are the following:
- Boron (B).
- Silicon (Yes).
- Germanium (Ge).
- Arsenic (Ar).
- Antimony (Sb).
- Tellurium (Te).
- Polonium (Po).
These elements are found, in the Periodic Table, distributed in a descending diagonal from boron to astatine (not including the latter), between columns 13, 14, 15, 16 and 17, thus dividing the entire table into two . The elements located in the right half are non-metallic and those located in the left half are metallic.
The metalloids they are more or less rare in the earth's crust. Some are very abundant, such as silicon, which usually appears forming compounds called silicates, or also arsenic, or boron, found as part of the mineral borax, since it does not exist in a free and pure state in nature.
Others, however, are quite rare, such as polonium, which appears as part of certain uranium minerals. Antimony, for example, is found in small percentages on planet Earth.
See also: Ionic bond
Characteristics of metalloids

Metalloids are very varied in terms of their appearance, that is, shape and color. Some are shiny and some are dull and many also present more than one allotropic state, that is, more than one presentation, depending on their molecular structure.
For example, arsenic can be gray, yellow or black, depending on its allotropic version. Silicon, likewise, can appear as a shiny solid crystal, or as a shapeless, brownish powder.
In any case, Most metalloids are electrical semiconductors that is, they conduct electricity, but less than metals, which are conductors. Even so, they are much better conductors than non-metal elements (which are usually insulators), which is why they have numerous industrial uses.
As with electricity, metalloids conduct heat much better than non-metallic elements but without reaching the high conductivity of metals.
Such an intermediate condition allows metalloids to react differently, depending on whether they are in the presence of a metal (in which case they will react as a non-metal) or a non-metal (then they will react as a metal). In general terms, they are quite reactive elements, rarely found in pure form in nature and have three or more electrons in their valence shell.
For that same reason, are usually toxic. Even some, such as arsenic, which are essential for the formation of vital molecules and are found in the body of living beings. In fact, poisoning from boron or arsenic itself is often fatal; while polonium, for example, is not only toxic, but highly radioactive.
Uses of metalloids
For the most part, semimetals are useful in the manufacture of electronic devices and other objects that use semiconductors, such as rectifiers, transistors, diodes, integrated circuits or even, in the case of silicon, for chips and microprocessors present in practically all the devices we use today.
However, being so varied, metalloids have other different uses, as part of pesticides, sealing materials or catalysts like some isotopes of Boron, for example, useful in the absorption of neutrons within nuclear power plants, thus functioning as agents for regulating atomic reactions.
Semimetals or metalloids?
Both terms are correct when naming this type of chemical elements: metalloids (that is, similar to metal) or semimetals (that is, they are not completely a metal). They can be used interchangeably.
Continue with: Metals
References
- “Semimetal” on Wikipedia.
- “Metaloids” on Wikipedia.
- “Metalloids in the Periodic Table” (video) in ChemEng IQA.
- “Metals, non-metals, metalloids, physical and chemical properties” (video) in Academia Internet.
- “Metalloid” in The Encyclopaedia Britannica.