We explain what combustion is, how it occurs and what the stages of the reaction are. Also, classification and examples.
What is combustion?
Combustion is a type of exothermic chemical reaction. It can involve matter in a gaseous state or in a heterogeneous state (liquid-gaseous or solid-gaseous). It generates light and heat in most cases, and is produced considerably quickly.
Traditionally, combustion is understood as a rapid oxidation process of certain fuel elements that is, made up mainly of hydrogen, carbon and sometimes sulfur. Furthermore, it necessarily takes place in the presence of oxygen.
In reality, combustions are redox reactions (reduction-oxidation) that can occur either in a controlled manner, as in internal combustion engines, or in an uncontrolled manner, as in explosions. These reactions, involve exchange of electrons between atoms of matter during the reaction.
Combustions almost always generate thermal and light energy and also produce other gaseous and solid substances, such as carbon dioxide (CO2) and water vapor, or solid residues of the fuel (the substance consumed in the reaction) and the oxidizer (the substance that promotes the reaction). The substances generated depend on the chemical nature of the reactants involved in combustion.
In this way, although in the traditional image of combustion there is always fire involved, it is possible that fire is not generated, since it is nothing more than a form of plasma (ionized gas) product of the release of heat from the chemical reaction of combustion, which to form depends on the conditions and reactants of each specific reaction.
See also: Enthalpy
How does combustion occur?
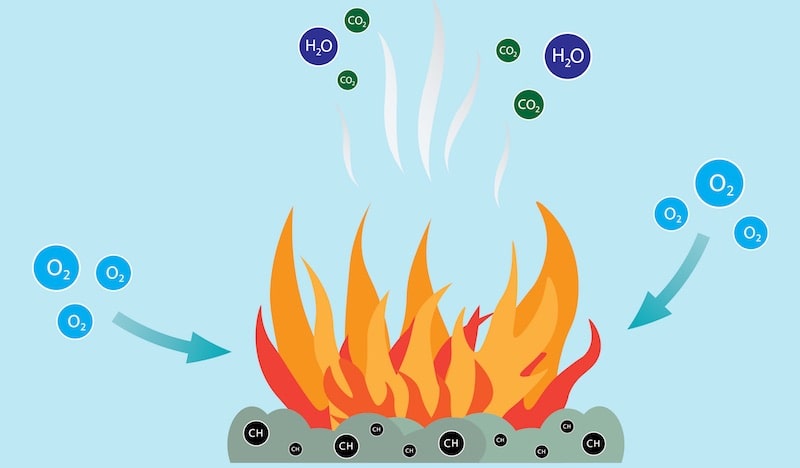
The combustions are a type of redox reaction that is, a reduction-oxidation reaction. This means that in them one reagent is oxidized (loses electrons), while the other is reduced (gains electrons).
In the case of combustion, the oxidizing agent (oxygen) obtains electrons from the reducing agent (fuel) or what is the same, the oxidizer (oxygen), obtains electrons from the fuel. This is generally given according to the following formula:
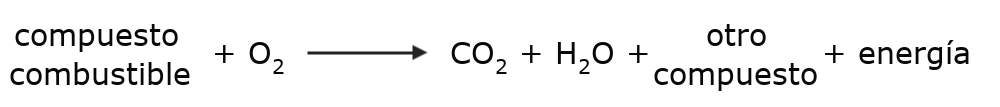
The fuel compounds may vary in each combustion reaction, depending on their nature, as well as the levels of energy generated. But carbon dioxide and water are produced in some way in all combustion.
Combustion types
There are three types of combustion:
- Complete or perfect combustions They are those reactions in which the fuel material is completely oxidized (consumed) and other oxygenated compounds are produced, such as carbon dioxide (CO).2) or sulfur dioxide (SO2), as the case may be, and water (H2EITHER).
- Stoichiometric or neutral combustions This is the name given to ideal complete combustions, which use just the right amounts of oxygen for their reaction and which generally occur only in the controlled environment of a laboratory.
- Incomplete combustions They are those reactions in which compounds that have not been completely oxidized (also called unburned) appear in the combustion gases. Such compounds can be carbon monoxide (CO), hydrogen, carbon particles, etc.
Combustion reaction
Combustion processes actually comprise a set of rapid chemical reactions that occur simultaneously. Each of these reactions can be called a stage or phase. The three fundamental stages of combustion are:
- Pre-reaction or first stage The hydrocarbons present in the fuel material decompose and begin their reaction with the oxygen in the air, forming radicals (molecularly unstable compounds). This starts a chain reaction of appearance and disappearance of chemical compounds where, usually, more compounds are formed than are broken down.
- Oxidation or second stage In this stage, most of the heat energy of the reaction is generated. As the oxygen reacts with the radicals from the previous stage, a process of violent displacement of electrons is generated. In the case of explosions, a high number of radicals leads to a massive and violent reaction.
- End of the reaction or third stage It occurs when the oxidation of the radicals is completed and the stable molecules that will be the products of combustion are formed.
Examples of combustion

Some simple examples of combustion in everyday life are:
- The lighting of a match/match It is the most emblematic case of combustion. When the match head (coated with phosphorus and sulfur) is scraped against a rough surface, it is heated by friction and triggers rapid combustion, which in turn produces a short flame.
- The ignition of a gas stove Domestic stoves operate by combustion of a hydrocarbon gas, usually a mixture of propane (C3h8) and butane (C4h10), which the device extracts from a pipe or a container. Placed in contact with air and provided with an initial charge of heat energy (such as a pilot light or a match), the gas begins its reaction; but to keep the flame burning, fuel must be supplied continuously.
- Strong bases and organic matter Most strong bases (hydroxides), such as caustic soda, caustic potash and other substances with extreme basic pH, generate violent oxidation reactions upon contact with organic matter. This means that we can burn ourselves through contact with these substances and even start fires with them, since these reactions are usually very exothermic.
- Internal combustion engines These devices are present in cars, boats and other vehicles that operate with fossil fuels such as diesel, gasoline or kerosene. They are an example of the use of controlled combustion. In them, the hydrocarbons in the fuel are consumed and small explosions are generated that, within the piston system, are transformed into movement, also producing polluting gases, which are released into the atmosphere.
Continue with: Endothermic reactions
References
- “Combustion” https://es.wikipedia.org/
- “Combustion as a process of energy transformation” in Ambientum, environmental encyclopedia. https://www.ambientum.com/
- “Combustion and types of combustion” http://www.expower.es/
- “The Combustion Process” at Auburn University. http://www.auburn.edu/
- “Combustion” https://www.grc.nasa.gov
- “Combustion (Chemical reaction)” https://www.britannica.com/





