We explain what a chemical change is and what its characteristics are. Also, examples and differences with a physical change.

What is a chemical change?
Chemical changes are a type of alteration in matter that modifies its chemical constitution that is, it alters its nature and not only its form. This means that chemical changes, also called chemical reactions or chemical phenomena, involve breaking and forming chemical bonds of chemical substances or compounds to form new substances or compounds.
Chemical reactions occur when two or more substances (called reactants) are chemically combined, changing their chemical structure in the process, and can consume (endothermic reactions) or release (exothermic reactions) energy, to generate two or more new substances ( called products). Some chemical reactions are dangerous for humans, as they can involve or produce toxic or corrosive compounds. Other reactions, such as certain exothermic reactions, can cause explosions.
In the chemical industry, many materials that we use in daily life are produced through controlled chemical reactions. Some reactions occur spontaneously and others must be generated by humans in industrial plants or chemical laboratories.
Chemical reactions require a stipulated time to happen, which varies depending on the nature of the reactants and the conditions in which the reaction occurs.
Thus, the factors that affect the rate of chemical reactions generally tend to be:
- Temperature increase Increasing temperature tends to increase the speed of chemical reactions.
- Increase in pressure Increasing pressure usually increases the speed of chemical reactions. This generally occurs when substances that are sensitive to changes in pressure react, such as gases. In the case of liquids and solids, changes in pressure do not cause significant changes in the rate of their reactions.
- State of aggregation in which the reactants are found Solids usually react more slowly than liquids or gases, although the speed will also depend on the reactivity of each substance.
- Use of catalysts They are substances that are used to increase the speed of chemical reactions. These substances do not intervene in the reactions, they only control the speed at which they occur. There are also substances called inhibitors, which are used in the same way but cause the opposite effect, that is, they slow down the reactions.
- Luminous energy (Light). Some chemical reactions are accelerated when light shines on them.
- Concentration of the reactants Most chemical reactions occur faster if they have a high concentration of their reactants.
See also: Product in chemistry
Examples of chemical change

Any chemical reaction is a perfect example of a chemical change, even those that occur in our bodies. Some examples are:
- The breathing It is a biological process of chemical change, in which oxygen is taken from the air and used to react with the glucose that we obtain from food, generating high levels of chemical energy (ATP) and amounts of carbon dioxide (CO2) waste, which must be expelled from the body.
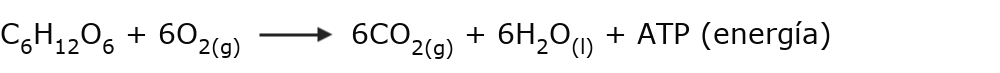
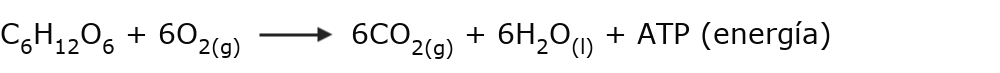
- Acid rain It occurs in environments where the atmosphere is highly polluted. It is usually the result of chemical changes that occur between the water stored in the clouds and other gases dispersed in the air, whose sulfur oxide or nitrogen oxide content generates sulfuric acid or nitric acid that fall along with rain.
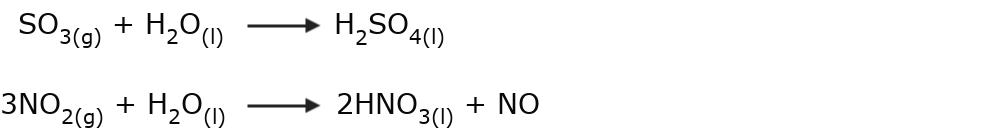
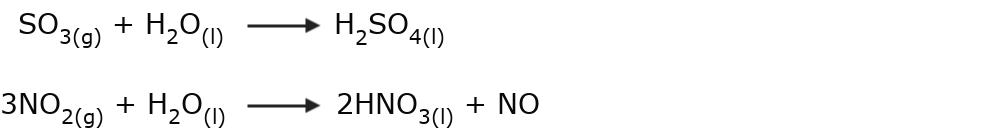
- The formation of salts The reaction that occurs inside batteries is produced between an acid and a metal. For example, batteries that use lead and sulfuric acid produce lead (II) sulfate, a white salt.
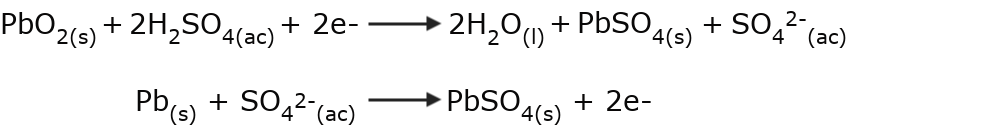
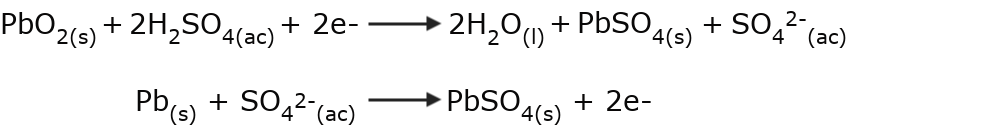
- The decomposition of ozone The ozone molecule is decomposed into oxygen molecules by the action of a certain type of light.


Chemical change and physical change

Physical changes in matter do not alter its composition That is, they do not modify the chemical structure of the substances, so through a physical change they cannot decompose or form substances. Physical changes only change physical properties of substances such as shape, density and states of aggregation (solid, liquid, gaseous). On the other hand, physical changes are usually reversible, since they alter the form or state of matter, but not its composition.
For example, by boiling water we can convert a liquid into a gas, but the resulting vapor is still composed of water molecules. On the contrary, if we freeze water, it becomes a solid state, but it still remains chemically the same substance. Another example is the liquefied gas we use in our lighters, which is usually butane (C4h10) or propane (C3h8) transformed to the liquid state by applying high pressures, but without altering its chemical composition.
Chemical changes alter the distribution and bonds of the atoms of matter causing them to combine in a different way, thus obtaining substances different from the initial ones. When a chemical change occurs, you always obtain the same amount of matter as you had at the beginning, even if it is in different proportions, since matter cannot be created or destroyed, only transformed.
For example, if we react water (H2O) and potassium (K), we will obtain two new substances: potassium hydroxide (KOH) and hydrogen (H2). This is a reaction that normally releases a lot of energy, therefore it is very dangerous.
References
- “Chemical reaction” https://es.wikipedia.org/
- “What is a chemical change?” https://www.tplaboratorioquimico.com/
- “What are chemical changes?” http://recursostic.educacion.es/
- “Physical and Chemical Changes” (video) on MooMooMath. https://www.youtube.com/
- “Physical and Chemical Changes: Chemistry for Kids” (video) in Free School. https://www.youtube.com/
- “Chemical Change Definition in Chemistry” https://www.thoughtco.com/





