We explain what redox reactions are, the types that exist, their applications, characteristics and examples of redox reactions.
What are redox reactions?
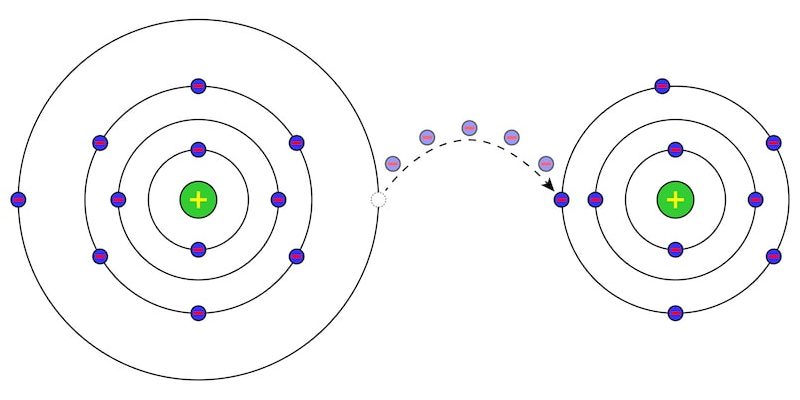
In chemistry, they are known as redox reactions, oxidation-reduction reactions or reduction-oxidation reactions. chemical reactions in which an exchange of electrons occurs between atoms or molecules involved.
This exchange is reflected in the change in the oxidation state of the reactants. The reactant that gives up electrons undergoes oxidation and the one that receives them, reduction.
The oxidation state indicates the number of electrons that an atom of a chemical element gives up or accepts when it is part of a chemical reaction. It can also be interpreted as the supposed electric charge that a certain atom would have if all its bonds with other atoms were completely ionic. It is also called oxidation number or valence.
The oxidation state is expressed in whole numbers, with zero being the oxidation state for neutral elements. Thus, it can take positive or negative values depending on the type of atom and the reaction in which it participates. On the other hand, some atoms have variable oxidation states depending on the reaction in which they are involved.
Knowing how to correctly determine the state or oxidation number of each atom in a chemical compound is essential to be able to understand and analyze redox reactions. There are certain rules that allow you to calculate their values:
- The oxidation number of neutral elements or molecules is zero. For example: solid metals (Fe, Cu, Zn…), molecules (O2N.2F.2).
- Ions composed of a single atom have their oxidation number equal to their charge. For example: Na+,Li+Ca2+Mg2+Faith2+Faith3+Cl–.
- Fluorine always has an oxidation state of -1 because it is the most electronegative element that exists (F–).
- Hydrogen always has oxidation number +1 (H+), with the exception of metal hydrides (potassium hydride, KH), where it has an oxidation number -1 (H–).
- Oxygen has an oxidation number -2, with some exceptions:
- When it forms compounds with fluorine it has an oxidation number of 2+. For example: oxygen difluoride (OF2).
- When it forms peroxides it has oxidation number -1 (O22-). For example: hydrogen peroxide (H2EITHER2), sodium peroxide (Na2EITHER2).
- When it forms superoxides it has oxidation number -½ (O2–). For example: potassium superoxide (KO2).
- The algebraic sum of the oxidation numbers of the atoms that constitute a neutral compound is zero.
- The algebraic sum of the oxidation numbers of the atoms that constitute a polyatomic ion is equal to the charge of the ion. For example: the sulfate anion (SO42-) has oxidation number -2, which is equal to the sum of the oxidation numbers of sulfur and oxygen, each multiplied by the amount of each atom in the compound, in this case, it has one sulfur atom and four oxygen atoms.
- The oxidation numbers of some chemical elements can vary depending on the neutral compound or ion of which they are a part. Then, it is possible to calculate the oxidation number of an atom in a compound in the following way:


Where No() means oxidation number, and the chemical element is found within the parentheses.
This way, In every redox reaction there are two types of reactants, one that gives up electrons and another that accepts them:
- An oxidizing agent. It is the atom that captures the electrons. In this sense, its initial oxidation state decreases, and a reduction is experienced. In this way, its negative electrical charge increases by gaining electrons.
- A reducing agent. It is the atom that gives up electrons and increases its initial oxidation state, undergoing oxidation. In this way, its positive electrical charge increases by giving up electrons.
Some chemical elements can be oxidized and reduced at the same time. These elements are called ampholytes and the process in which this happens is called ampholytes.
redox reactions are one of the most common chemical reactions in the universe since they are part of the processes of photosynthesis in plants and respiration in animals, which allow the continuity of life.
Characteristics of redox reactions
Redox reactions are found around us every day. The oxidation of metals, the combustion of gas in the kitchen or even the oxidation of glucose to obtain ATP in our body are some examples.
In most cases, redox reactions release a significant amount of energy
Typically, each redox reaction is composed of two steps or half-reactions. In one of the half-reactions, oxidation occurs (the reactant is oxidized) and in the other, reduction occurs (the reactant is reduced).
The total redox reaction, which is obtained as a result of algebraically combining all the half-reactions, is usually called the “global reaction.” It is important to note that when half-reactions are combined algebraically, both the mass and the charge must be adjusted. That is, the number of electrons given up during oxidation must be the same as the number of electrons gained during reduction, and the mass of each reactant must be equal to the mass of each product.
For example:
- Reduction half-reaction Reduction of copper by capturing two electrons. It decreases its oxidation state.
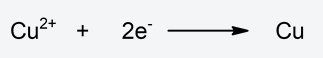
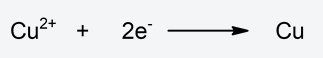
- Oxidation half-reaction Oxidation of iron by losing two electrons. It increases its oxidation state.
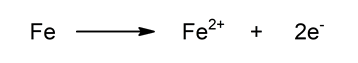
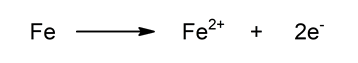
Overall reaction: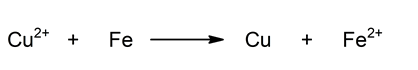
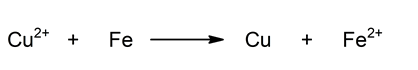
Types of redox reactions
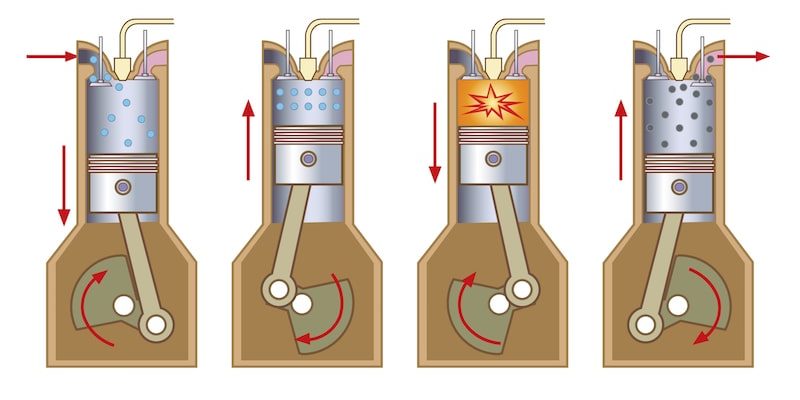
There are different types of redox reactions, with different characteristics. The most common types are:
- Combustion Combustions are redox chemical reactions that release a significant amount of energy in the form of heat and light. These reactions are rapid oxidations that release a lot of energy. The energy released can be used in a controlled way to generate movement in car engines. An element called oxidizer (which is reduced and oxidized to the fuel) and a fuel element (which is oxidized and reduced to the oxidizer) participate in these reactions. Some examples of fuels are gasoline and the gas we use in our kitchens, while the best known oxidizer is gaseous oxygen (O2).
- Oxidation of metals They are slower reactions than combustions. They are commonly described as the degradation of certain materials, especially metallic ones, due to the action of oxygen on them. It is a worldwide known and daily phenomenon, especially in coastal towns, where salts in the environment accelerate (catalyze) the reaction. That is why a car, after taking us to the beach, must be cleaned of all traces of salt water.
- Disproportion. Also known as dismutation reactions, they present a single reagent that is reduced and oxidized at the same time. A typical case of this is the decomposition of hydrogen peroxide (H2O2).
- Simple scrolling. Also called “single substitution reactions,” they occur when two elements exchange their respective places within the same compound. That is, one element replaces another in its exact place in the formula, balancing their respective electrical charges with other atoms as appropriate. An example is what happens when a metal displaces hydrogen in an acid and salts are formed, such as when batteries in a device break down.
Examples of redox reactions
Examples of redox reactions are very abundant. We will try to give an example of each of the types described above:
- The combustion of octane Octane is a hydrocarbon component of gasoline used to power our car engines. When octane reacts with oxygen, octane is oxidized and oxygen is reduced, releasing a large amount of energy as a result of this reaction. This released energy is used to generate work in the engine, also producing carbon dioxide and water vapor in the process. The equation that represents this reaction is:
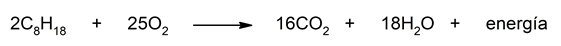
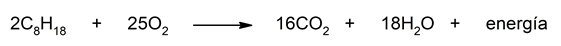
- The decomposition of hydrogen peroxide. It is a dismutation reaction in which hydrogen peroxide is decomposed into its constituent elements, water and oxygen. In this reaction, oxygen is reduced, decreasing its oxidation number from -1 (H2EITHER2) up to -2 (H2O), and is oxidized by increasing its oxidation number from -1 (H2EITHER2) to 0 (O2).
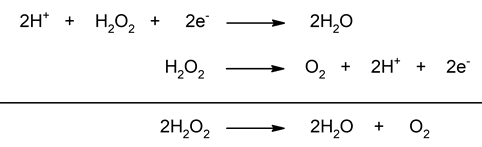
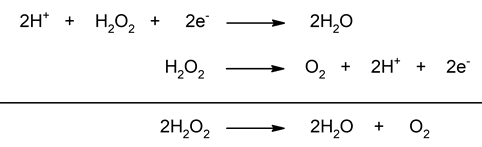
- Displacement of silver by copper. It is a simple displacement reaction in which you can see how when you immerse a fragment of metallic copper in a solution of silver nitrate, the color of the solution turns blue and a thin layer of metallic silver is deposited on the copper fragment. In this case, part of the metallic copper (Cu) is transformed into the Cu ion2+as part of copper (II) nitrate (Cu(NO3)2), whose solution has a nice blue color. On the other hand, part of the Ag cation+which is part of silver nitrate (AgNO3), is transformed into metallic silver (Ag) that is deposited.


- Reaction of zinc with dilute hydrochloric acid It is a simple displacement reaction in which the hydrogen from HCl(ac) It is displaced by zinc to form a salt.
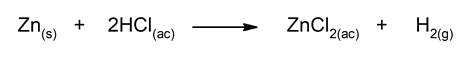
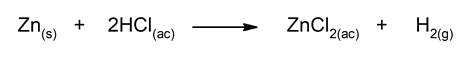
- Iron oxidation. Metallic iron oxidizes when it comes into contact with oxygen in the air. This is seen in daily life when a layer of brown rust forms on iron objects when they are exposed to the air for a long time. In this reaction, metallic iron (Fe), which has an oxidation state of 0, is transformed into Fe3+, that is, its oxidation state increases (it oxidizes). For this reason, it is said intuitively or colloquially: iron rusts.
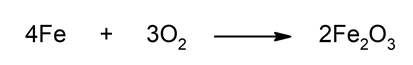
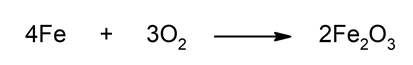
Industrial applications

The industrial applications of redox reactions are endless. For example, combustion reactions are ideal for producing work that is used to generate movement in the large engines used in power plants to produce electricity.
The process consists of burning fossil fuels to obtain heat and produce water vapor in a boiler, then this water vapor is used to drive large engines or turbines. On the other hand, combustion reactions are also used to operate the engines of motor vehicles that use fossil fuels, such as our cars.
On the other hand, Redox substitution and displacement reactions are useful to obtain certain elements in a state of purity which is not often seen in nature. For example, silver is extremely reactive. Although it is rare to find it pure in the mineral subsoil, a high degree of purity can be obtained through a redox reaction. The same happens when it comes to obtaining salts and other compounds.
Continue with: Metabolism
References
- “Reduction-oxidation” https://es.wikipedia.org/
- “Redox reactions: chemistry” (video) in Educatina. https://www.youtube.com/
- “Oxidation-reduction (redox) reactions” https://es.khanacademy.org/
- “Redox Reactions: Crash Course” (video) at CrashCourse. https://www.youtube.com/
- “Oxidation-reduction reaction” https://www.britannica.com/