We explain what acids and bases are, their characteristics, indicators and examples. Also, what is the neutralization reaction.
What are acids and bases?
A acid It is that chemical substance capable of giving up protons (H+) to another chemical substance. A base It is that chemical substance capable of capturing protons (H+) of another chemical substance.
However, there are two fundamental theories to explain what acids and bases are: the Arrhenius theory and the Brönsted-Lowry theory.
According to Arrhenius' theory:
An acid is a substance that gives up protons (H+) in aqueous solution. That is, it is a neutral substance, which when dissolved in water dissociates into its ions according to the following representative reaction:
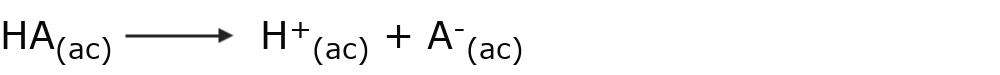
For example: hydrochloric acid (HCl)
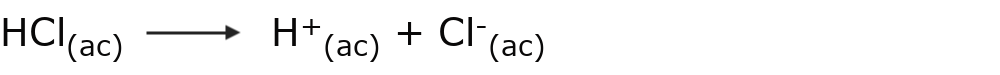
A base is a substance that gives up OH ions.– in aqueous solution. For example: sodium hydroxide (NaOH)
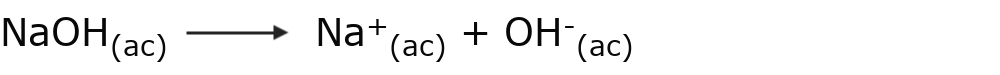
This theory has its limitations, since according to it these compounds are only defined in aqueous solution and not in other media. Furthermore, it does not explain compounds such as ammonia (NH3), which is a base, but since it does not have OH– in its composition, it does not meet the definition of Arrhenius base.
For all this, a new theory was needed that better explained the concepts of acid and base. So later Brönsted and Lowry developed a new theory, which includes Arrhenius' principles but is not only intended in aqueous solution, and is therefore much broader.
According to the Brönsted-Lowry theory:
According to this theory, an acid is a chemical substance that is capable of giving up protons (H+) to another chemical substance and a base is that chemical substance that is capable of capturing protons (H+) of another chemical substance.
According to this theory, an acid-base reaction is an equilibrium that can be expressed as:
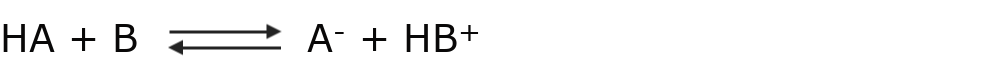
Where HA behaves like an acid, since it gives up a proton H+ to stay as A–. On the other hand, B behaves like a base, since it captures a proton H+ to become HB+.
Some substances can behave like acids and bases at the same time and are said to be amphoteric. This depends on the environment they are in or who they react with. An example of this type of substance is water:
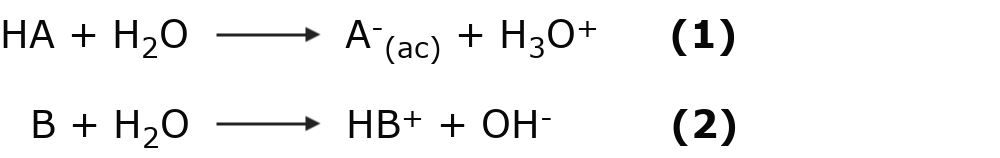
In the first equation, water picks up a proton H+behaving like a base and becoming H3EITHER+. While in the equation (2), water gives up a proton H+behaving like an acid and becoming OH–.
Apparently in both theories, acids and bases have different proportions of hydrogen ions (H+). This determines its acidity (in the case of acids) or its alkalinity or basicity (in the case of bases).
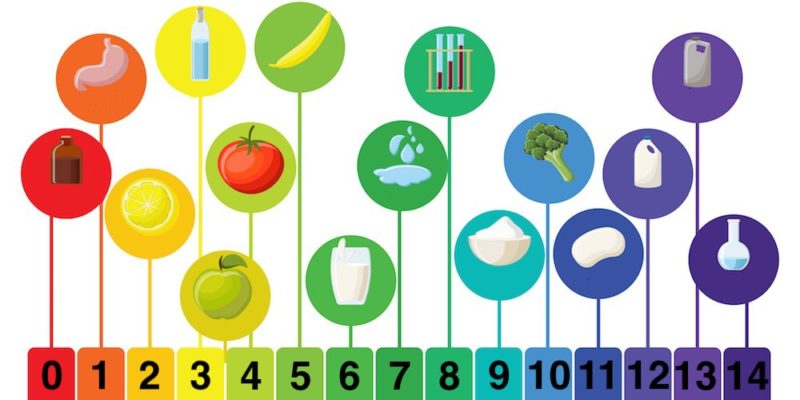
The pH is the magnitude used to measure the acidity or alkalinity of a solution, that is, it indicates the concentration of hydrogen ions present in it.
- Acids Substances with pH from 0 to 6.
- Neutrals Substance with pH 7 (water).
- Bases/alkalines Substances with pH from 8 to 14.
The lower the pH of a substance, the higher its degree of acidity. For example, pure HCl has a pH close to 0. On the other hand, the higher the pH of a substance, the greater its degree of alkalinity. For example, caustic soda has a pH of 14.
Characteristics of acids and bases
Both acids and bases can exist as liquids, solids or gases. On the other hand, they can exist as pure or diluted substances, retaining many of their properties.
The pH difference is the most noticeable feature of each. When the pH value of a compound reaches one of its extremes, it means that this compound is highly dangerous for most matter, both organic and inorganic.
Acids and bases have different physical characteristics:
acids
- They have a sour flavor (for example: acid present in various citrus fruits).
- They are highly corrosive and can cause chemical burns to the skin or respiratory damage if their gases are inhaled.
- They are good conductors of electricity in aqueous solutions.
- They react with metals producing salts and hydrogen.
- They react with metal oxides to form salt and water.
Bases
- They have a characteristic bitter flavor.
- They are good conductors of electricity in aqueous solutions.
- They are skin irritants: they dissolve skin oil and can destroy organic matter due to their caustic effect. Their breathing is also dangerous.
- They have a soapy touch.
- They are soluble in water.
Acids and bases in everyday life

The presence of acids and bases in our daily lives is abundant. For example, Inside the batteries of our electronic devices there is usually sulfuric acid. Therefore, when they spoil and their contents are poured into the device, they react with the metal of the electrodes and create a whitish salt.
Also there are mild acids that we handle daily such as acetic acid (vinegar), acetylsalicylic acid (aspirin), ascorbic acid (vitamin C), carbonic acid (found in carbonated soft drinks), citric acid (found in citrus fruits), or hydrochloric acid ( the gastric juice that our stomach secretes to dissolve food).
Regarding the bases, Baking soda is used in baking, as a deodorant, and in various heartburn remedies. Other commonly used bases are sodium carbonate (detergent), sodium hypochlorite (cleaning bleach), magnesium hydroxide (laxative), and calcium hydroxide (building lime).
Indicators of acids and bases
The way to distinguish between an acidic compound and a basic one is by measuring its pH value. Currently there are numerous methods to measure the pH of a substance.
- Using acid-base indicators. Indicators are compounds that change color when the pH of the solution in which they are found changes. For example, phenolphthalein is a liquid that turns pink if added to a base and becomes colorless if added to an acid. Another example is litmus paper, which is immersed in a solution and if it turns red or orange it will be an acidic substance and if it turns a dark color it will be a basic solution.
- Using a potentiometer or pH-meter. There are electronic devices that directly give us the pH value of a solution.
Neutralization reaction
The neutralization reaction or (acid-base reaction) is a chemical reaction that occurs when these two types of compounds are mixed obtaining in exchange a salt and a certain amount of water. These reactions are usually exothermic (they generate heat) and their name comes from the fact that the properties of acid and base cancel each other out.
To classify neutralization reactions, it is important to know the types of acids and bases.
- Strong acid. It is an acid that when in aqueous solution is completely ionized, that is, it is completely transformed into the ions that make up its molecule. For example: HCl(ac)HBr(ac)H.2SW4(ac).
- Strong base It is a base that when in aqueous solution is completely ionized, that is, it is completely transformed into the ions that make up its molecule. For example: NaOH(ac)LiOH(ac)K.O.H.(ac).
- weak acid It is an acid that when in aqueous solution is partially ionized, that is, it is not completely transformed into the ions that make up its molecule. Therefore, the concentration of ions in solution of this type of acid is lower than in a strong one. For example: citric acid, carbonic acid (H2CO3)
- Weak base. It is a base that when in aqueous solution partially ionizes. That is, it is NOT completely transformed into the ions that make up its molecule. Therefore, the concentration of ions in solution of this type of base is lower than in a strong one. For example: ammonia (NH3), ammonium hydroxide (NH4OH)
Neutralization reactions can occur in four ways, depending on the properties of their reactants:
- A strong acid and a strong base The most abundant reactant will remain in solution with respect to the other. The pH of the resulting solution will depend on which reagent is in greater proportion.


- A weak acid and a strong base. A solution of basic pH will be obtained, the base will remain in the solution.


- A strong acid and a weak base The acid is neutralized and an acid proportion will remain in solution, depending on the degree of concentration of the acid. The pH of the resulting solution is acidic.


- A weak acid and a weak base. The result will be acidic or basic depending on the concentrations of its reactants.
Examples of acids and bases
acids
- Hydrochloric acid (HCl)
- Sulfuric acid (H2SW4)
- Nitric acid (HNO3)
- Perchloric acid (HClO4)
- Formic acid (CH2EITHER2)
- Bromic acid (HBrO3)
- Boric acid (H3B.O.3)
- Acetic acid (C2h4EITHER2)
Bases
- Caustic soda (NaOH)
- Calcium hydroxide (Ca(OH)2)
- Ammonia (NH3)
- Sodium bicarbonate (NaHCO3)
- Potassium hydroxide (KOH)
- Sodium hypochlorite (NaClO)
- Calcium fluoride (CaF2)
- Barium hydroxide (Ba(OH)2)
- Iron (III) hydroxide (Fe(OH)3)
Continue with: Chemical formula
References
- “Acids and bases” https://es.khanacademy.org/
- “Acids and bases in biology” https://www.ck12.org/
- “Neutralization of acids and bases” at the University of the Basque Country. http://www.ehu.eus/
- “Acids and bases” https://www.encyclopedia.com/
- “Acids and Bases” in Lumen Learning. https://courses.lumenlearning.com/
- “Acids and Bases” https://www.bbc.co.uk/





