We explain what hydrolysis is and what this chemical reaction consists of. Also, the types of hydrolysis that exist.

What is hydrolysis?
Hydrolysis is a chemical reaction in which water molecules (H2O) are divided into their component atoms (hydrogen and oxygen). In turn, in the hydrolysis process, the atoms that make up the water molecules begin to form chemical bonds with the substance that reacts with the water. Hydrolysis is a very important reaction, since water is the most used solvent worldwide.
The specific name of this reaction comes from the Greek words hydro (“water”) and lysis (“rupture”), from which it follows that it is a form of breakdown of a given solute molecule when it reacts with water. In terms of organic chemistry, this is the exact opposite process of the condensation reaction, which is the combination of two organic molecules, in which a product and a water molecule are obtained.
There are various forms of hydrolysis, depending on the substances that react with water:
- Acid-base hydrolysis In this reaction, water splits into a hydroxyl ion (OH–) and a proton (H+), which is immediately hydrated to form a hydronium ion (H3EITHER+). Thus, pure water manifests this reaction spontaneously.
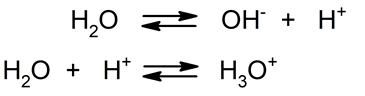
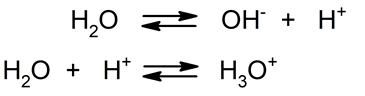
When certain substances are added to water, the balance of the previous reaction can be modified. For example, if we add salts, depending on their solubility, their anions or cations can combine with OH ions.– and H.3EITHER+which can cause the pH of the final solution to vary. Thus, there are four classifications for acid-base hydrolysis depending on the type of salt added to the water: - Hydrolysis of strong acid-strong base salt When a salt from a strong acid and a strong base is diluted in water, almost no hydrolysis occurs, so the dissociation equilibrium of water is not altered. The pH in this case will be neutral. For example:
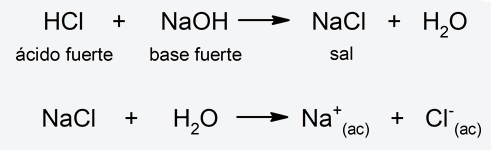

- Weak acid-strong base salt hydrolysis The anion of the salt (from the weak acid and the strong base) and a proton from water combine, releasing hydroxyl ions, due to which the resulting pH will be basic. For example:
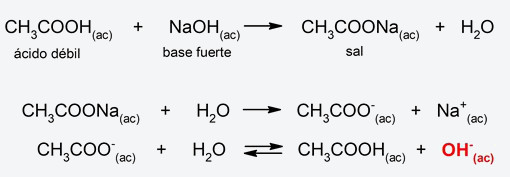

- Strong acid-weak base salt hydrolysis The cation of the salt (from the strong acid and the weak base) gives up a proton to water to form a hydronium ion (H3EITHER+), due to which the resulting pH will be acidic. For example:
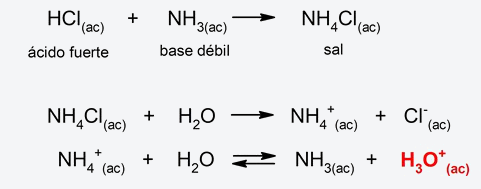

- Weak acid-weak base salt hydrolysis. The salt cation (from the weak base) combines with water, releasing a hydronium ion (H3EITHER+) and the anion of the salt (from the weak acid) combines with water releasing a hydroxyl ion (OH–). The resulting pH will depend on the amounts of hydronium and hydroxyl ions produced. If more H ion is produced3EITHER+ what OH ion– the pH will be acidic, and if more OH ion is produced– what ion H3EITHER+the pH will be basic. On the other hand, if the quantities of both ions produced are equal, the resulting pH will be neutral. For example:
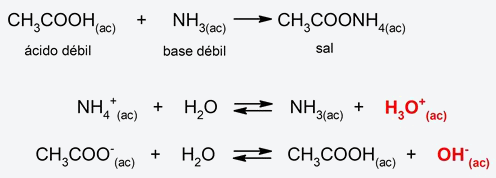

- Hydrolysis of amides and esters. In these types of organic substances, hydrolysis can occur in an acidic or basic medium. In the case of esters, they are hydrolyzed in acidic (1) and basic (2) medium, generating carboxylic acids and alcohols. The ester hydrolysis process is also called saponification (hydrolysis of triglycerides to obtain soaps). On the other hand, amides generally hydrolyze in an acidic medium, decomposing into amines and carboxylic acids. For example:
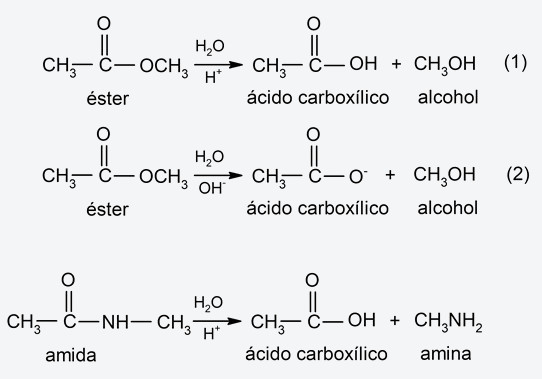

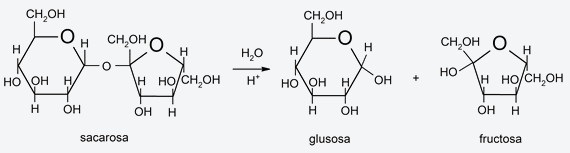
- Hydrolysis of polysaccharides Polysaccharides (sugars) can be hydrolyzed and broken down (breaking their glycosidic bonds, which are bonds between monosaccharides to form polysaccharides) into simpler polysaccharides, disaccharides, or monosaccharides. In the hydrolysis process, a hydrogen from the water molecule bonds to the oxygen at the end of a sugar molecule, while the hydroxyl bonds to the end of the rest. The hydrolysis of polysaccharides is a process regularly carried out by life forms.

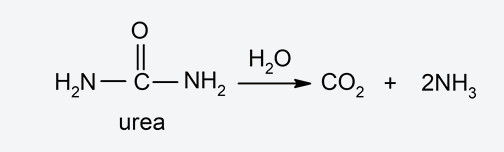
- Enzymatic hydrolysis It is the hydrolysis that occurs in the presence of enzymes (organic compounds that generally increase the speed of chemical reactions) called hydrolases. For example, urea amidohydrolase is an enzyme that is involved in the hydrolysis of urea:

See also: Inorganic chemistry
References
- Hydrolysis of polysaccharides. Biochemistry and Molecular Biology online. Dr Eggar Vázques Contreras
- “Hydrolysis” https://es.wikipedia.org/