We explain what analytical chemistry is and what this branch of chemistry focuses on. Also, the analytical methods it uses.

What is analytical chemistry?
Analytical chemistry is called a branch of chemistry that focuses on understanding matter that is, the analysis of the materials that make up a sample, using experimental or laboratory methods.
analytical chemistry can be classified into quantitative and qualitative analytical chemistry. Quantitative analytical chemistry is used to determine the quantity, concentration or proportion of one or more components in a sample, that is, it is concerned with quantifying the matter.
Qualitative analytical chemistry is used to know what the components of a sample are, that is, it is concerned with identifying each component of the sample. On the other hand, analytical chemistry is also used for the separation of the components of a sample. Generally, the substance in question (the one you want to identify or quantify) is called analyte.
The knowledge that gave rise to analytical chemistry arose from the modern idea of the chemical composition of matter, which emerged in the 18th century.
An important milestone in the development of this discipline was the understanding of the correlation between the physical properties of matter and its chemical composition. In this, the study of spectroscopy, electrochemistry and polarography were fundamental.
However, the invention of methods of chemical analysis that would allow a more complete understanding of the subject would advance along with scientific and technological development, so that the general characteristics of the field of analytical chemistry would be defined only in the 20th century.
Analytical chemistry uses the following analytical methods to understand matter:
Quantitative methods
- Volumetric methods Known as titration or titration, they are quantitative methods in which a reagent whose concentration is known (titrant substance) is used to determine that of another reagent whose concentration is unknown (analyte or substance to be analyzed in the sample), through a chemical reaction. In titrations, indicators are generally used to mark the end point of the reaction. There are different types of degrees:
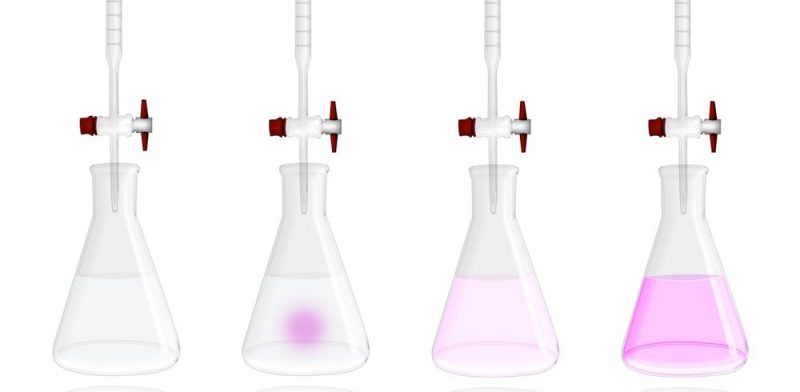
- Acid-base titrations They are those in which an acid is reacted with a base using an acid-base indicator. Typically, the base is placed in a burette (chemical container used to measure volumes) and a known volume of the acid is placed in an Erlenmeyer flask with a few drops of phenolphthalein (indicator) added. Phenolphthalein takes on a pink color in a basic medium and is colorless in an acidic medium. Then the method consists of adding the base to the acid until the final solution turns pink, which means that the reaction between the acid and the base has reached its end point. An instant before reaching the end point, the reaction reaches its equivalence point, which is where the amount of substance in the titrant is equal to the amount of substance in the analyte. If the stoichiometry in the reaction is 1:1, that is, the same amount of analyte substance and titrant react, the following equation can be used to determine the amount of analyte:


Where:
(x) is the known concentration of the substance x, expressed mol/L or equivalent units.
V(X) is the volume of the substance x dispensed from the burette, expressed in L or equivalent units.
(AND) is the unknown concentration of the analyte ANDexpressed in mol/L or equivalent units.
V(Y) is the volume of the substance AND contained in the Erlenmeyer, expressed in L or equivalent units.
It is important to clarify that, although this equation is widely used, it often varies depending on the type of degree used. - Redox titrations The basis is the same as in acid-base titrations, but in this case there is a redox reaction between the analyte and an oxidizing or reducing solution, as the case may be. The indicator used can be a potentiometer (equipment to measure potential difference) or a redox indicator (compounds that have a defined color in each of their oxidation states).
- Complex formation titrations They consist of the complex formation reaction between the analyte and the titrant substance.
- Precipitation titrations They consist of the formation of a precipitate. They are very specific and the indicators used are very particular to each reaction.
- Acid-base titrations They are those in which an acid is reacted with a base using an acid-base indicator. Typically, the base is placed in a burette (chemical container used to measure volumes) and a known volume of the acid is placed in an Erlenmeyer flask with a few drops of phenolphthalein (indicator) added. Phenolphthalein takes on a pink color in a basic medium and is colorless in an acidic medium. Then the method consists of adding the base to the acid until the final solution turns pink, which means that the reaction between the acid and the base has reached its end point. An instant before reaching the end point, the reaction reaches its equivalence point, which is where the amount of substance in the titrant is equal to the amount of substance in the analyte. If the stoichiometry in the reaction is 1:1, that is, the same amount of analyte substance and titrant react, the following equation can be used to determine the amount of analyte:
- Gravimetric methods Quantitative method that consists of measuring the weight of a material or substance before and after making any changes to it. The instrument to perform the measurement is generally an analytical balance. There are several gravimetric methods:
- Precipitation It consists of the formation of a precipitate, so that when weighed, its quantity in the original sample can be calculated using stoichiometric relationships. The precipitate can be collected from the solution in which it is found by filtration. To apply this method, the analyte must be poorly soluble and be chemically well defined.
- Volatilization It consists of volatilizing the analyte to separate it from the sample. Then the analyte is recovered by absorbing it into some material, this material is weighed, and the weight gain will be due to the incorporation of the analyte, whose weight will be calculated by the difference in weight of the absorbent material before and after having absorbed the analyte. This method can only be applied when the analyte is the only volatile substance in the sample.
- Electrodeposition It consists of a redox reaction where the analyte is deposited on an electrode forming part of a compound. The electrode is then weighed before and after the redox reaction, in this way the amount of analyte deposited can be calculated.
More advanced instrumental methods:
- Spectrometric methods Devices are used to measure the behavior of electromagnetic radiation (light) in contact with the substance or compound being analyzed.
- Electroanalytical methods Similar to spectrometry, but electricity is used instead of light to measure the electrical potential or the electric current transmitted by the substance to be analyzed.
- Chromatographic methods Chromatography is a method of separation, characterization and quantification of complex mixtures. It is used to separate one or several components of a mixture and at the same time identify them and calculate their concentration or quantity in the sample, that is, quantify them. The chromatographic method basically consists of a stationary phase and a mobile phase that are part of a piece of equipment or structure that is used to analyze the sample. The stationary phase is immobile and consists of a substance that adheres to some system designed generally in the form of a column and the mobile phase is a substance (liquid or gaseous) that flows through the stationary phase. The separation of the components (analytes) occurs according to the affinity of each one of them for the stationary phase or for the mobile phase, which will depend on various chemical and physical properties (of each one or both phases). There are different types of chromatography depending on the substances used as mobile and stationary phases, the conditions imposed on the method and the designs of the chromatographic equipment. For example, in the following image you can see the separation of the different components of a mixture that was injected into a chromatographic column. You can see the different colors of each component as they descend through the stationary phase that fills the column:


See also: Law of Conservation of Matter