We explain what an exothermic reaction is and its differences from an endothermic reaction. Also, examples of this chemical reaction.

What is an exothermic reaction?
An exothermic reaction is one that When it occurs, it releases energy in the form of heat or light to the environment. When this type of reaction occurs, the products obtained have lower energy than the initial reactants.
Enthalpy is a quantity that defines the flow of thermal energy in chemical processes that occur at constant pressure. Furthermore, this magnitude represents the exchange of energy between a thermodynamic system and its environment. The variation of this magnitude (ΔH) in a chemical reaction is used to classify it as endothermic or exothermic.
ΔH>0 endothermic reaction.
ΔH<0 exothermic reaction.
Exothermic reactions are very important in biochemical sciences. Through reactions of this type, living organisms obtain the energy necessary to sustain life in a process called metabolism.
Most exothermic reactions are oxidation and when they are very violent they can generate fire, as in combustion. Other examples of these reactions are the transitions of matter from one state of aggregation to another of lower energy, such as from gas to liquid (condensation), or from liquid to solid (solidification).
In fact, many exothermic reactions are dangerous to health because the energy released is abrupt and uncontrolled, which can cause burns or other damage to living beings.
See also: Principle of conservation of energy
Differences between exothermic and endothermic reactions

In every chemical reaction, energy is conserved. This constitutes the law of conservation of energy: energy is neither created nor destroyed, only transformed.
In endothermic reactions, energy is absorbed to transform reactants into products. In this type of reaction, the bonds of the molecules that make up the reactants are broken to form new components. This bond breaking process requires the energy in question. An example of this is the process of water electrolysis, where electrical energy is supplied to the water molecule to break it and transform it into its constituent elements.
On the other hand, in exothermic reactions, the reactants release chemical energy contained in the bonds that form their molecules. The energy released can be in the form of heat or light.
Examples of exothermic reaction
Some known exothermic reactions are:
- Combustion. It is a very rapid oxidation reaction that occurs between materials called fuels and oxygen. Fuels are mainly made up of carbon, hydrogen and, in some cases, sulfur. Examples of fuels are methane gas, gasoline and natural gas. This reaction releases large amounts of heat, which can lead to fire.
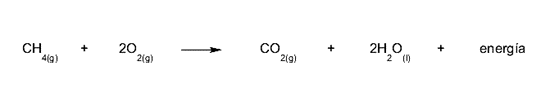
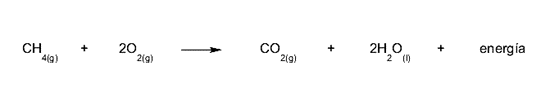
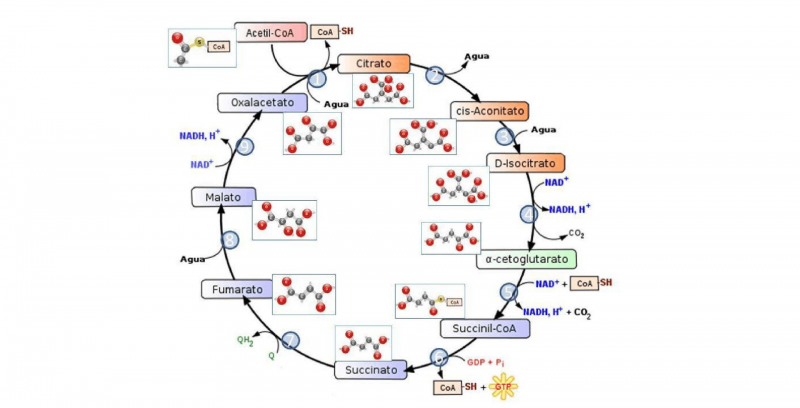
- The oxidation of glucose. This is the reaction that animals carry out to obtain metabolic energy: we take oxygen from respiration and use it to oxidize sugars, breaking the glucose molecule into simpler molecules (glycolysis) and obtaining ATP molecules as a reward, rich in chemical energy.
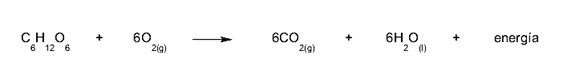
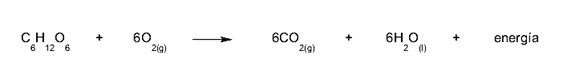
- The mixture of potassium and water. Potassium is a powerful desiccant that, when mixed with water, releases hydrogen and enormous amounts of energy in an explosion. This occurs with all alkali metals, although not always with the same amount of energy released.
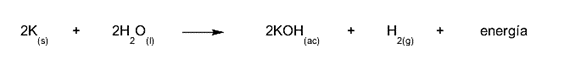
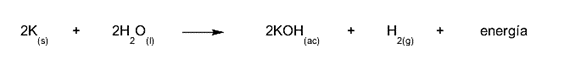
- The formation of ammonia. To form ammonia (NH3) nitrogen (N) is reacted2) and hydrogen (H2), which means obtaining a molecule that is less energetic than the molecules put into reaction. That difference in energy must be released, and it occurs as an increase in temperature (heat).
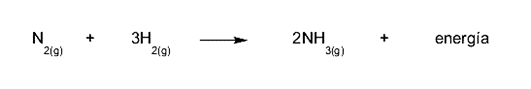
References
- “Endothermic and exothermic reaction” on the CCH Academic Portal of the National Autonomous University of Mexico (UNAM)
- “Exothermic reaction” in EcuRed, Knowledge with everyone and for everyone.
- “Obtaining ammonia” in LaGuía.