We explain what a battery is and how this device works. Also, the types of batteries that exist and what a battery is.

What is a battery?
An electric battery, also called an electric cell or accumulator, is a device made up of electrochemical cells capable of converting the chemical energy inside into electrical energy. Thus, the batteries generate direct current and, in this way, They serve to power different electrical circuits depending on its size and power.
The batteries have been fully incorporated into our daily lives since their invention in the 19th century and its massive commercialization in the 20th century. The development of batteries goes hand in hand with the technological advancement of electronics. Remote controls, watches, computers of all kinds, cell phones and a huge group of contemporary devices use batteries as a source of electrical power, which is why they are manufactured with various powers.
The batteries have a load capacity determined by the nature of their composition and it is measured in ampere-hours (Ah), which means that the battery can deliver one ampere of current over one continuous hour of time. The higher its charging capacity, the more current it can store inside.
Finally, the short life cycle of most commercial batteries has turned them into a powerful pollutant of water and soil, since once their life cycle has expired they cannot be recharged or reused, and are discarded. After their metal cover oxidizes, the batteries release their chemical content into the environment and alter their composition and pH.
See also: Electrical conductivity
How does a battery work?

The fundamental principle of a battery is the oxidation-reduction (redox) reactions of certain chemicals one of which loses electrons (is oxidized) while the other gains electrons (is reduced), and can return to its initial configuration given the necessary conditions: the application of electricity (charge) or the closure of the circuit (discharge).
The batteries contain chemical cells that have a positive pole (anode) and a negative pole (cathode) as well as electrolytes that allow electrical flow to the outside. These cells convert chemical energy into electrical energy, through a reversible or irreversible process, depending on the type of battery, which once complete, exhausts its capacity to receive energy. There are two types of cells:
- Primary Those that, once the reaction has occurred, cannot return to their original state, thus exhausting their capacity to store electrical current. They are also called non-rechargeable batteries.
- Secondary Those that can receive an application of electrical energy to restore their original chemical composition, and can be used numerous times before being completely exhausted. They are also called rechargeable batteries.
Battery types
There are many types of batteries, depending on the elements used in their manufacture, such as:
- Alkaline batteries Commonly disposable. They use potassium hydroxide (KOH) as an electrolyte. The chemical reaction that produces energy occurs between zinc (Zn, anode) and manganese dioxide (MnO2cathode). They are extremely stable batteries, but short-lived.
- Lead-acid batteries Common in vehicles and motorcycles. They are rechargeable batteries that when charged have two lead electrodes: a lead dioxide cathode (PbO2) and a spongy lead (Pb) anode. The electrolyte used is sulfuric acid (H2SW4) in aqueous solution. On the other hand, when the battery is discharged, the lead is in the form of lead (II) sulfate (PbSO4) deposited in metallic lead (Pb). So, during the initial charge the PbSO4 is reduced to Pb on the negative plates, and PbO is formed2 in the positive ones. In this process, lead is oxidized and reduced at the same time. On the other hand, during discharge the PbO2 is reduced to PbSO4 and Pb is oxidized to also produce PbSO4. These two processes can be repeated cyclically until the PbSO crystals4 They become too large so they lose chemical reactivity. This is the case where it is colloquially said that the battery has sulfated and must be replaced with a new one.
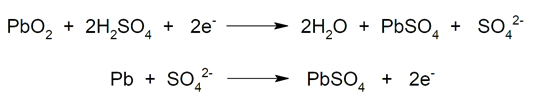
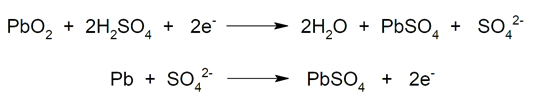
- Nickel batteries Very low cost but lousy performance, they are some of the first to be manufactured in history. In turn, they gave rise to new batteries such as:
- Nickel-iron (Ni-Fe). They consisted of thin tubes wound with sheets of nickel-plated steel. The positive plates had nickel (III) hydroxide (Ni(OH)3) and in the negative ones iron (Fe). The electrolyte used is potassium hydroxide (KOH). Although they lasted very long, they were discontinued due to their low performance and high cost.
- Nickel-cadmium (Ni-Cd) They are composed of a cadmium (Cd) anode and a nickel (III) hydroxide cathode (Ni(OH)3), and potassium hydroxide (KOH) as electrolyte. These batteries are perfectly rechargeable, but have low energy density (just 50Wh/kg). In addition, they are used less and less due to their high memory effect (reduction in battery capacity when we charge incompletely) since cadmium is very polluting.
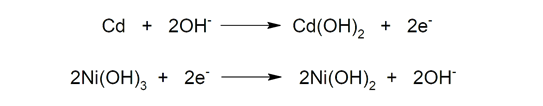
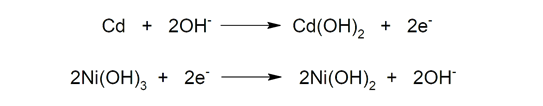
- Nickel-hydride (Ni-MH) They use nickel oxyhydroxide (NiOOH) for the anode and a metal hydride alloy as the cathode. They have a greater charging capacity and less memory effect compared to Ni-Cd batteries, and they do not affect the environment since they do not have Cd (very polluting and dangerous). They were the pioneers in being used for electric vehicles, since they are perfectly rechargeable.
- Lithium ion batteries (Li-ION) They use a lithium salt as an electrolyte. They are the batteries most used in small electronics, such as cell phones and other portable devices. They stand out for their enormous energy density, added to the fact that they are very light, have a small size and good performance, but they have a maximum life of three years. Another advantage they have is their low memory effect. Furthermore, when overheated they can explode, since their elements are flammable, so their production cost is high because safety elements must be incorporated.
- Lithium polymer (LiPo) batteries They are a variation of ordinary lithium batteries, they have better energy density and a better discharge rate, but they have the disadvantage of being unusable if they lose their charge below 30%, so it is essential not to let them discharge completely. They can also overheat and explode, so it is very important to never wait too long to look at the battery, or always keep it in a safe place away from flammable substances.
Battery and battery

The terms stack and battery In this context they are synonyms and come from the early days of human manipulation of electricity. The first accumulators consisted of groups of cells or metal discs to increase the current initially supplied, and which could be arranged in two ways: one on top of the other, forming a stackor one next to the other, in the form of battery.
It should be clarified, however, that in many Spanish-speaking countries only the term is used batteryand it is preferred accumulator for other electrical devices, such as capacitors, etc.





