We explain what Van der Waals forces are and in what cases they manifest themselves. Also, why do they have such a name and their characteristics.
What are Van der Waals forces?
A certain type of force is known as Van der Waals forces or Van der Waals interactions. attractive or repulsive intermolecular forces different from those that generate atomic bonds (ionic, metallic or covalent of the reticular type) or the electrostatic attraction between ions and other molecules.
Before mentioning the different types of Van der Waals forces, it is important to understand what chemical polarity is. Chemical polarity is a property of molecules that tend to separate electrical charges in their structure. It is a property closely related to intermolecular forces (such as Van der Waals), solubility, and melting and boiling points. Depending on polarity, molecules can be classified into:
- polar molecules They are made up of atoms with very different electronegativities. The atom with the highest electronegativity attracts the electrons from the bond and is left with a negative charge density on it. On the other hand, the atom with the lowest electronegativity will have a positive charge density on it. This distribution of charges will eventually lead to the formation of a dipole (system of two charges of opposite sign and equal magnitude).
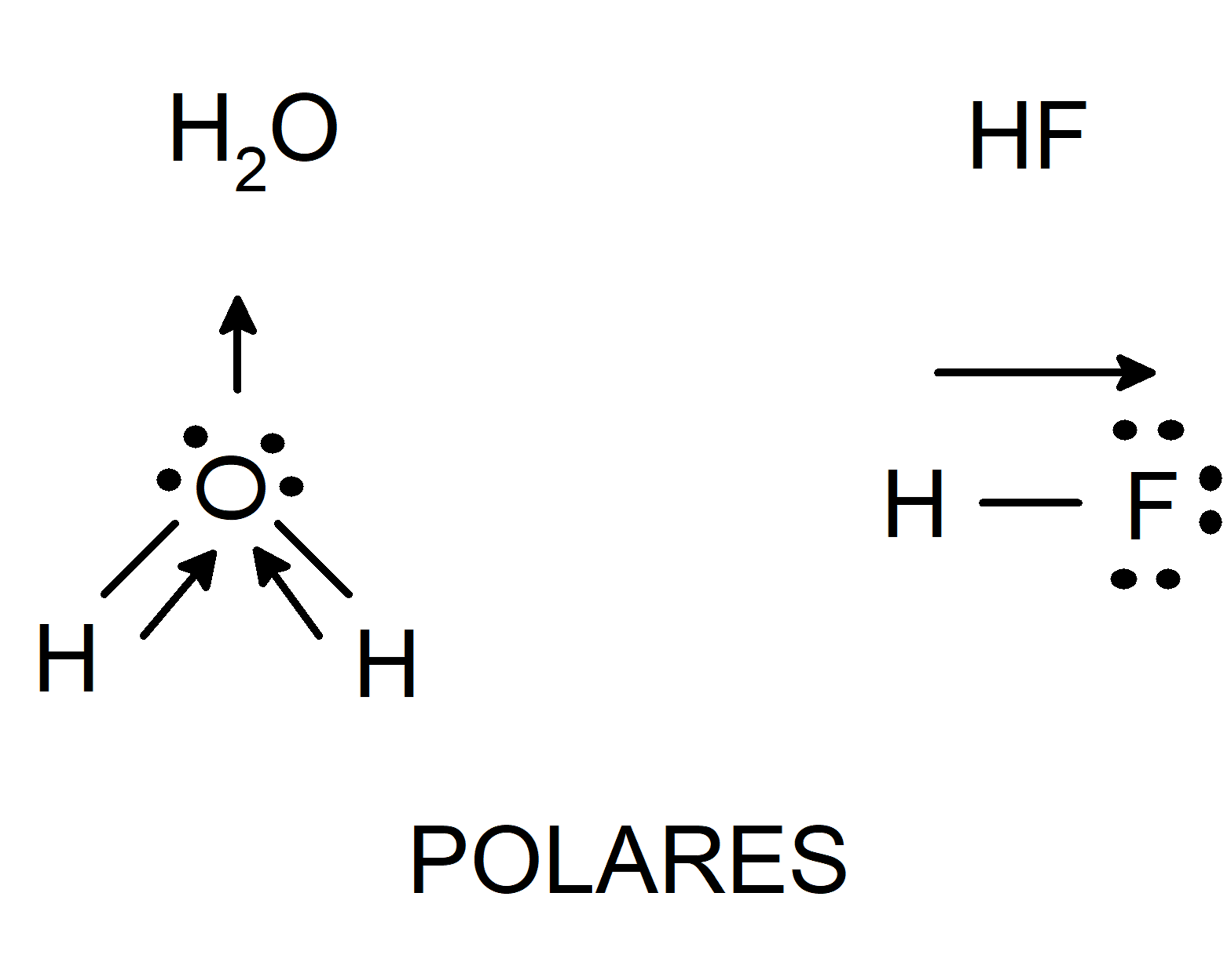
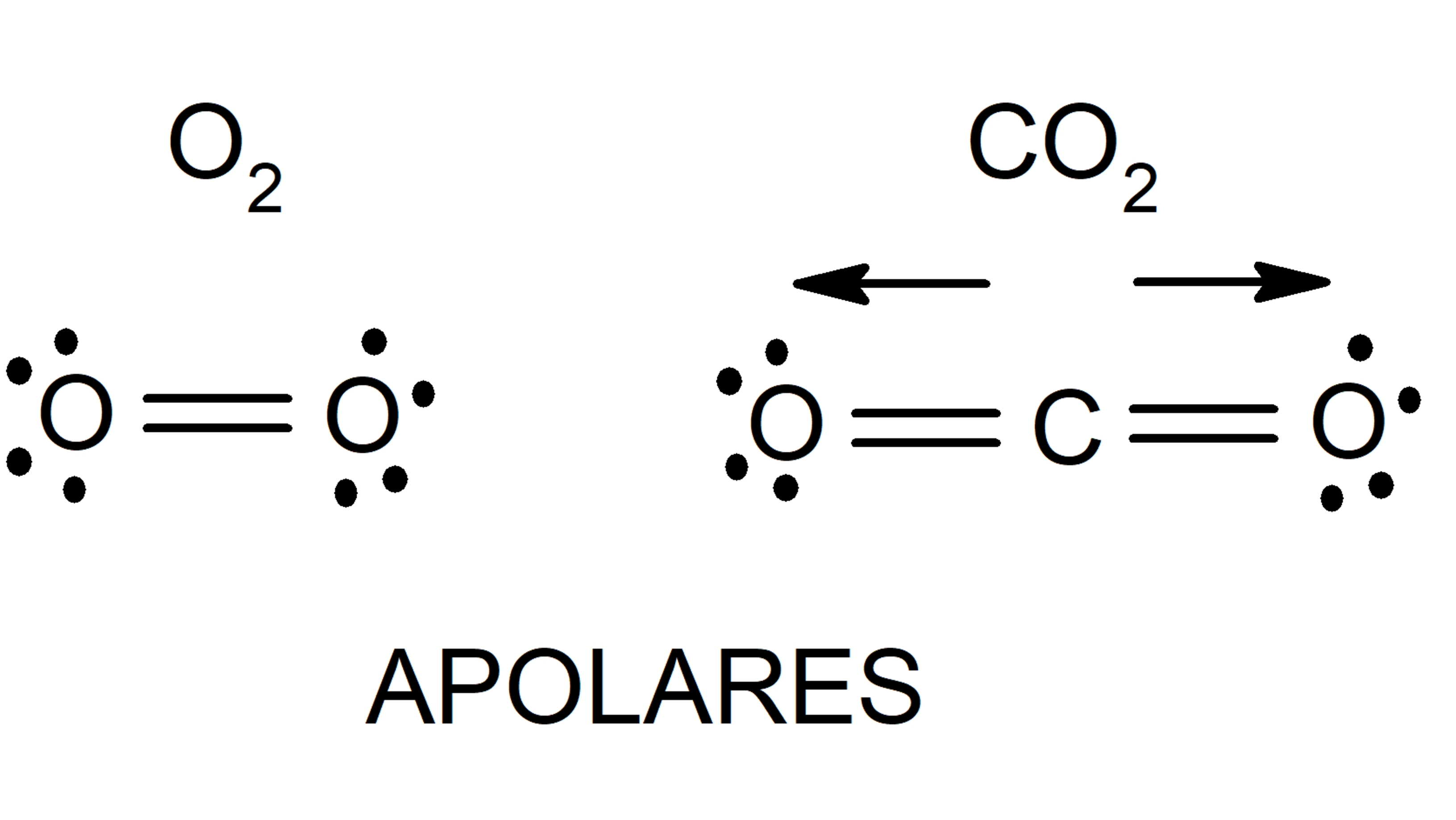
- Nonpolar molecules They are made up of atoms with equal electronegativity, so all atoms attract the electrons of the bond in the same way. A factor that also determines the polarity of a molecule is molecular symmetry. There are molecules composed of atoms of different electronegativity, but which are not polar. This occurs because when the different charge densities of the parts of the molecule are added, they cancel out and result in a zero dipole moment.

So, Van der Waals forces manifest themselves in three particular ways:
- Keesom forces of attraction (dipole-dipole interactions) They are interactions between polar molecules, that is, permanently polarized. Thus, these molecules have a positive pole (with positive charge density 𝛅+) and a negative one (with negative charge density 𝛅–), and are oriented so that the positive pole approaches the negative pole.
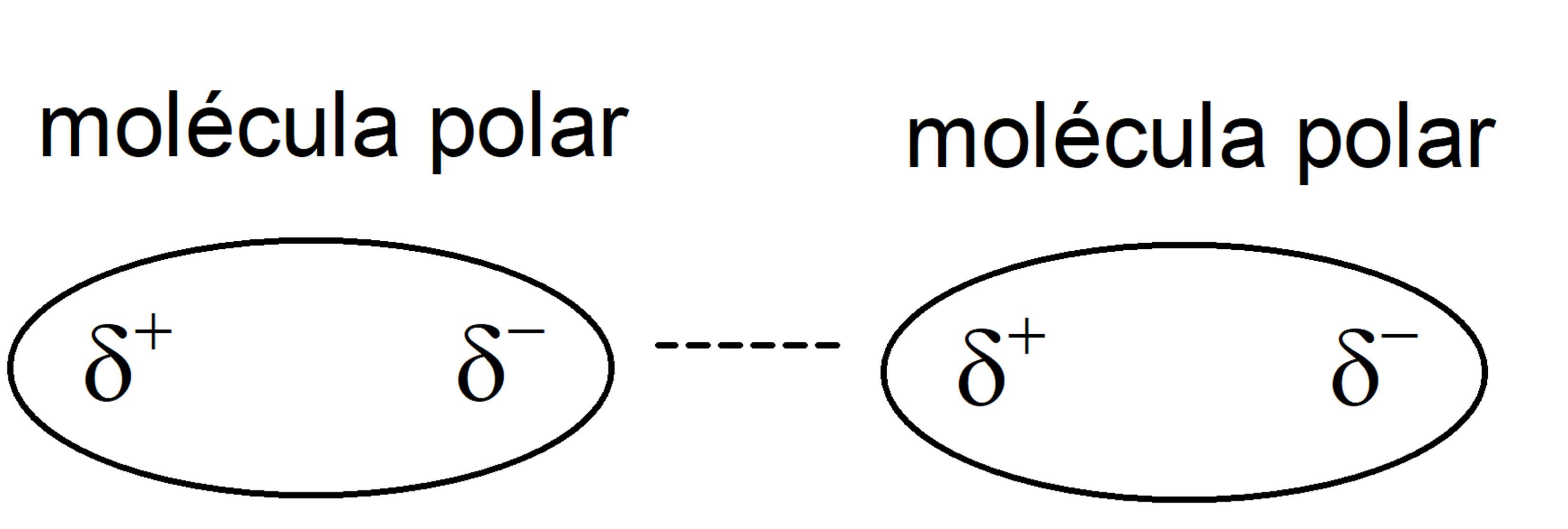
- Debye attractive forces (permanent dipole-induced dipole interactions) They take place between a polar molecule and another nonpolar one, but which has an induced polarity. In this type of interaction, the dipole induces a transient dipole in the nonpolar molecule.


- London dispersion forces (induced dipole-induced dipole) They are interactions that occur between nonpolar molecules. The movement of electrons in these molecules induces transient dipoles, which causes some attraction between them. They are very weak interactions.
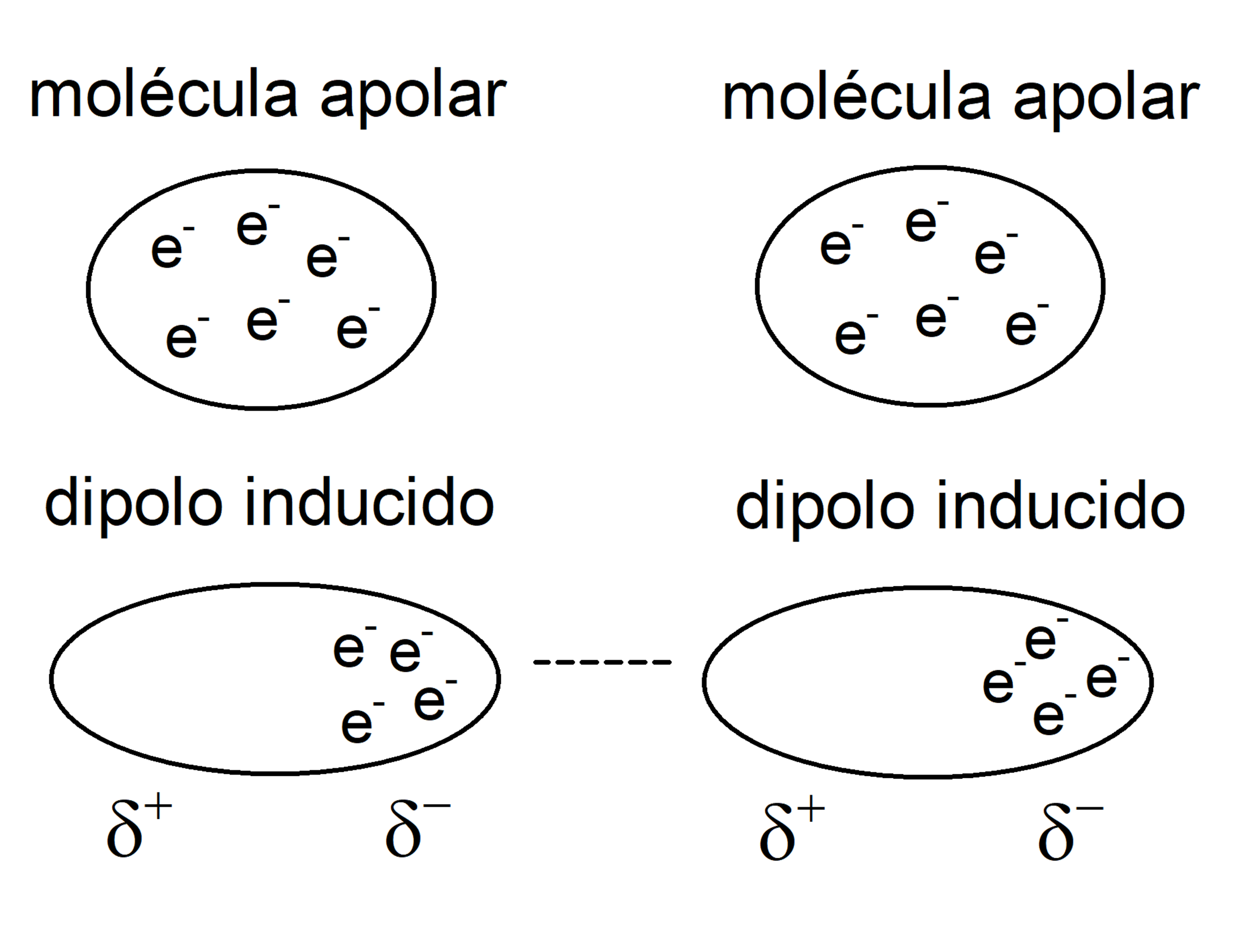
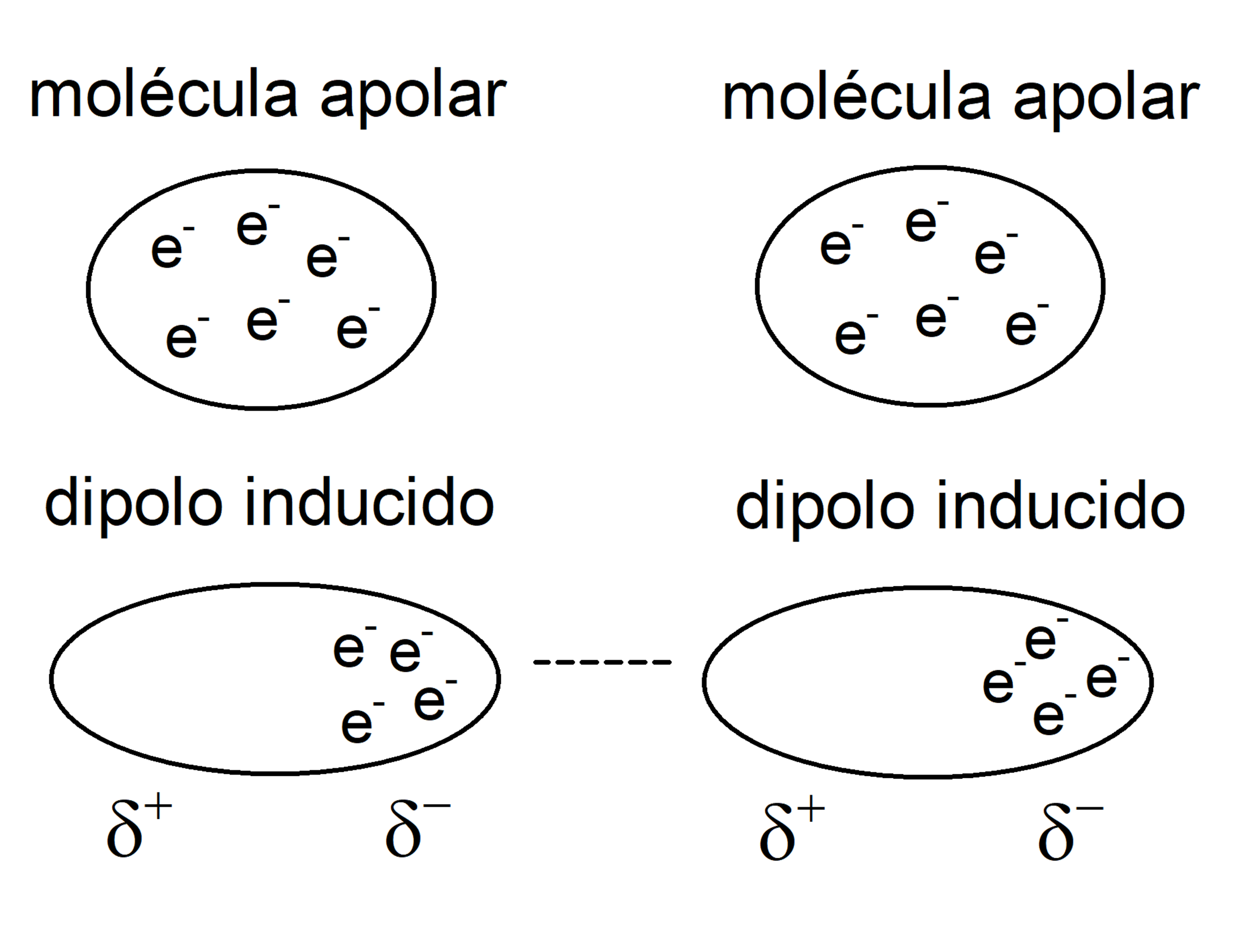
All of these intermolecular forces are known as Van der Waals forces, a name that pays tribute to Dutch physicist Johannes Diderik van der Waals (1837-1923), the first to propose its effects on the equations of state of a gas (known as the Van der Waals Equation) in 1873. For this discovery he was awarded the Nobel Prize in Physics in 1910.
See also: Potential energy
Characteristics of Van der Waals Forces

Van der Waals forces are generally weak compared to ordinary chemical bonds which does not prevent them from being fundamental for various fields of physics, biology and engineering. Thanks to them many chemical compounds can be defined.
Van der Waals forces grow with the length of the nonpolar end of a substance since they are caused by correlations between the fluctuating polarizations between atoms, molecules or nearby surfaces, a consequence of quantum dynamics.
They present anisotropy, that is, their properties vary depending on the orientation of the molecules: whether they are attractive or repulsive often depends on this.
These forces are the weakest that occur between molecules in nature: only 0.1 to 35 kJ/mol of energy is required to overcome them. However, are crucial for the formation of proteins.