We explain what endothermic reactions are and some examples of them. Also, what are exothermic reactions.

What are endothermic reactions?
Endothermic reactions are chemical reactions that require the supply of heat energy to occur. For the reactants to transform into products, these reactions absorb heat, which causes the products obtained to have higher energy levels than the initial reactants.
Enthalpy is a quantity that defines the flow of thermal energy in chemical processes that occur at constant pressure. Furthermore, this magnitude represents the exchange of energy between a thermodynamic system and its environment. The variation of this magnitude (ΔH) in a chemical reaction is used to classify the reaction as endothermic or exothermic.
ΔH>0 endothermic reaction.
ΔH<0 exothermic reaction.
These reactions are commonly used in the chemical ice and cooling industry, since can be generated in controlled environments to remove heat from environments or other substances. Some of its applications were replaced with the cold generated by cooling equipment.
See also: Principle of conservation of energy
Examples of endothermic reactions

Some examples of endothermic reactions are:
- The production of ozone in the atmosphere This reaction is driven by ultraviolet radiation from the Sun, oxygen molecules (O2) are converted into ozone (O3), absorbing energy from said radiation in the process.


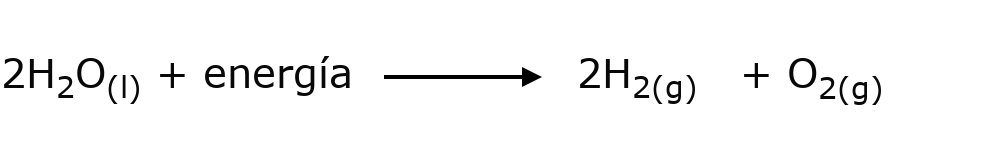
- The electrolysis of water To separate the hydrogen (H) and oxygen (O) that make up water (H2O) it is necessary to add electrical energy in a procedure known as electrolysis, in which both types of atoms respond to the poles generated by the added electric current, their chemical bond is broken and energy is consumed.

- Photosynthesis. The plant nutrition process occurs through a series of chemical reactions that break down carbon dioxide (CO2) environmental in the presence of water and sunlight. This series of reactions needs to consume energy to occur.
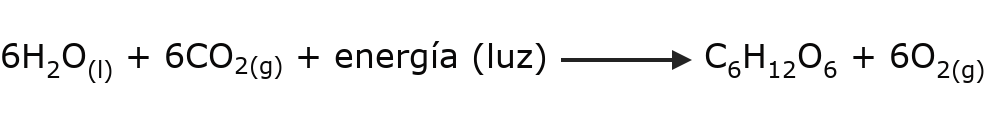
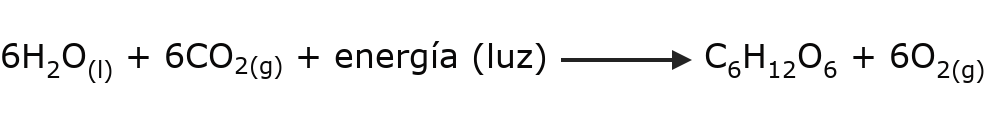
- Obtaining iron (II) sulfide This compound is obtained in a laboratory after reacting iron and sulfur. For this reaction to occur, it is necessary to supply heat energy using a burner (or a boiler if industrial conditions are involved).


Exothermic reactions

An exothermic reaction is one that When it occurs, it releases energy in the form of heat or light to the environment. When this type of reaction occurs, the products obtained have lower energy than the initial reactants. The enthalpy change for this type of reaction is less than zero (ΔH<0).
are examples of this type of reactions all forms of combustion and of oxidation as is the case of gasoline or fossil fuels, which when burned in the presence of oxygen release an amount of energy much higher than that initially introduced (the engine spark). The same occurs in phase changes of matter from the gaseous state to the liquid, or from the liquid to the solid.
References
- “Endothermic and exothermic reaction” on the CCH Academic Portal of the National Autonomous University of Mexico (UNAM). https://e1.portalacademico.cch.unam.mx/
- “Exothermic reaction” at https://www.ecured.cu/
- “Obtaining ammonia” at https://quimica.laguia2000.com/