We explain what carbon dioxide is and why it is important. Carbon dioxide and climate change. Also, carbon cycle.

What is carbon dioxide?
When talking about carbon dioxide, carbon dioxide or CO2 (from its chemical formula: CO2), reference is made to a colorless gas soluble in water whose molecules are composed of one carbon atom and two oxygen atoms, joined by covalent double bonds.
The CO2 It constitutes approximately 0.04% of the gases present in the Earth's atmosphere. It's a gas essential for life as we know it and it is present in numerous organic compounds, including hydrocarbons (natural gas, oil, etc.) or the air that aerobic living beings exhale (that is, that we breathe). The biological importance of CO2 It lies mainly in the fact that plants need it to carry out photosynthesis, as well as certain types of cyanobacteria for their energy production processes.
In the presence of constant pressure, carbon dioxide is a gas, but it is also can be forced to become liquid by increasing the pressure (through the process of liquefaction) or even in solid form, forming the so-called “dry ice” or dry ice.
The highest concentration of this gas on the planet is, however, in the atmosphere, dissolved among many other gases that make up the air. It is also found in hot springs, volcanoes and carbonate rocks, which when diluted in water or some acids release this gas. It is produced daily as a by-product of natural processes, such as respiration, the decomposition of organic matter or combustion (for example, in forest fires) and in the fermentation of sugars. It is also generated artificially, through the burning of fossil fuels and numerous industrial processes.
The CO2 It can also be found outside our planet: The atmospheres of Venus and Mars have shown abundant presence of this gas which makes up 95% of them.
See also: Air pollution
Uses of CO2
In principle, carbon dioxide is an extremely useful substance for man, who has been able to give it the following uses:
- In the food industry, it is injected into drinks (soft drinks) to give effervescence.
- It is part of the compounds present in fire extinguishers, since CO2 It is not fuel.
- It is frequently used as a refrigerant (in gas or as ice) and in the creation of special effects, such as artificial fog.
- It is part of the gases used to form laser beams.
- In medicine, it is used as a contrast agent or as a gas to insufflate in laparoscopies, as well as in aesthetic treatments.
carbon cycle
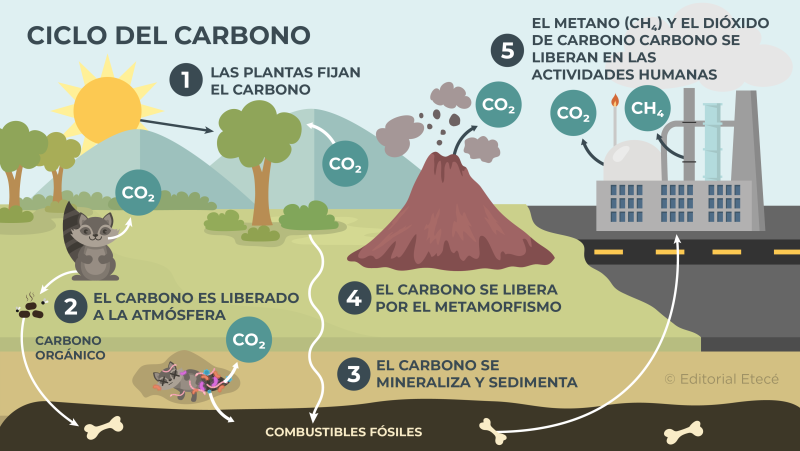
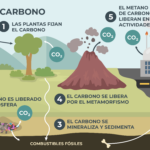
The CO2 on our planet is part of a biogeochemical cycle that exchanges carbon between the layers of the atmosphere, sea water and deposits on land. This allows carbon atoms to be reused and life to be sustainable on the planet.
Thus, the carbon present in methane (CH4) and CO2 Atmospheric passes through photosynthesis to plants, and also to water when diluted in raindrops that then run off to the ocean, where it forms small amounts of carbonic acid. This is where respiration and microbial decomposition cycles intervene, releasing new CO2 in gaseous form to the atmosphere.
Continue in: Carbon cycle
CO2 and climate change

Despite being naturally present in the atmosphere, carbon dioxide is, along with other compounds, a greenhouse gas. This means that it contributes to forming a gaseous layer in the atmosphere that prevents heat radiation and increases the temperature of the planetary surface which leads to gradual climate changes whose effects living beings suffer.
Everything indicates that the increase in CO levels2 in the atmosphere, a product of human industrial activity sustained since the 17th century (burning of hydrocarbons, metallurgy, mass fermentation, concrete manufacturing, etc.), would have noticeably unbalanced the carbon cycle, accumulating much more of this gas in the atmosphere which it is possible to get rid of naturally. To get an idea, the CO2 atmospheric in 1750 was 0.028% and at the beginning of the 21st century it is at 0.037%.
This increase in gas also slowly increases the planet's temperature by a few degrees and, although it may not seem like it, it has catastrophic effects on the climate by altering the delicate balance of the water cycle, ocean currents and heat distribution. This has consequences as serious as the creation of new deserts ; the melting of the poles and the perennial snows, thus increasing the level of the oceans; floods and torrential rains that ruin cities and crops, and cause land displacement; and even more extreme climatic seasons: colder winters and more intense summers.
As if that were not enough, the increase in CO2 atmospheric has an impact on the present in the oceans producing more carbonic acid and modifying the pH of the seas, which gradually become more acidic and less suitable for life.
The set of these processes and consequences is known as climate change and currently There is a whole debate regarding what measures to take to stop it, prevent it and even reverse it which requires a joint effort from the entire international community.