We explain what the methods are for separating mixtures and we define each of these methods with some simple examples.

What are mixture separation methods?
It is known as mixture separation methods or phase separation methods to the different physical procedures that allow two or more components of a mixture to be separated. The components of the mixture retain their identity and chemical properties after separation.
For these mechanisms to work, These must be mixtures in which the components retain their identity and there have been no chemical reactions that permanently alter its properties or give rise to new substances.
For separation methods to be applied, properties such as boiling point, density or size must be preserved in the components of the mixture.
Instead, these methods They work in both homogeneous and heterogeneous mixtures since they do not involve any change in the identity of the components, which can thus be recovered more or less as they were before the mixture was made. Depending on the method applied, the original components will be achieved with greater or lesser purity.
See also: Aggregation states of matter
Decantation
Decantation is a method used to separate liquids that do not dissolve in each other (such as water and oil) or solids insoluble in a liquid (such as water and sand).
It consists of the use of an ampoule or a separatory funnel where the mixture is allowed to rest until the densest ingredient settles and goes to the bottom. The valve is opened and it is let out, closing it in time so that the less dense ingredient remains inside. This method is usually used as a first step towards obtaining purer substances.
Continue in: Decantation
Filtration
Filtration is a useful method to separate insoluble solids from liquids. It consists of the use of a filter (filter paper, filter stones, etc.) that allows the liquid to pass through a porous medium and retains the solid elements.
This is how the water filters in our homes work, or the filter paper where we pour the solid coffee before adding hot water. The water (which contains the finer coffee particles) passes through the paper, and the coarser coffee particles are retained in it.
Continue in: Filtration
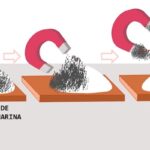
Magnetic separation
Magnetic separation consists of phase separation according to their magnetic potential. Some substances respond to magnetic fields and others do not, and according to this difference a magnet or electromagnet is applied to the mixture, which allows one component to be attracted and leave the other intact (for example, iron fragments in soil, mercury in water , pieces of metal in water).
Continue in: Magnetic separation
Sifting

Sieving operates in a similar way to filtering, but between solid substances of different sizes (such as gravel and sand, salt and popcorn, or rice and pebbles).
A net or sieve is used, whose holes allow the smaller fragments to pass through and retain the larger ones. Depending on the material, it can be used as a first step in obtaining pure substances or as a definitive step.
Continue in: Sifting
Distillation
The distillation allows you to separate soluble liquids from each other but that have different boiling points (like water and alcohol). The difference between the boiling points of the components to be separated by this method should be approximately 80 ºC.
The procedure consists of Pour the mixture into a container and heat it, controlling the temperature so that only the lowest boiling point component evaporates, and is taken through a conduit (called a distillation column) to another container, this time refrigerated. There it will condense and return to its original phase.
The liquids obtained in this way are known as distillates (distilled water, distilled alcohol).
Continue in: Distillation
Crystallization
Crystallization is an ideal method to separate solids dissolved in liquids (salt in water, sugar in water). It consists of evaporating the liquid until crystals of the dissolved solid are obtained at the bottom of the container. For example, this is how sea salt is obtained. Depending on the speed of evaporation, the crystals will be larger or smaller.
Continue in: Crystallization
Floatation

Flotation is the opposite case of decantation and consists of allow the lower density solid phase to float in the liquid and then remove it manually or using a sieve. The perfect example of this is the swimming pool cleaning procedure.
Chromatography
Chromatography is a method used to separate complex mixtures that do not respond to any other separation method. It uses capillarity as a principle: a process that allows the advancement of a substance through a specific medium. The two phases of the mixture are thus identified as the mobile phase (the one that advances) and the stationary phase (the one that advances).
The operation of this separation depends on the affinity of the components of the mixture for both phases, and according to this affinity, the separation will be faster or slower. For example, when spilling coffee on a cloth, the coffee moves forward, occupying a large amount of surface area.
Currently there are different chromatography methods:
- paper chromatography The stationary phase is made up of a strip of filter paper and the mobile phase is made up of a solvent that contains the sample to be separated. A few drops of the solvent containing the sample are placed on one end of the paper and we wait for the liquid to advance. It is then allowed to dry, and if the different components of the sample have different colors, their different positions on the paper can be observed.
- Thin layer chromatography The stationary phase is composed of an absorbent material adhered to a plate that can be made of glass, aluminum or another material. The mobile phase is a liquid that will act as an eluent. The procedure consists of placing the sample on the plate and then immersing a part of it in the eluent. The components will be separated by difference in affinity between the eluent and the component adhered to the plate.
- Column chromatography The stationary phase consists of a solid absorbent material that is placed as a filler in a glass column (although currently there are columns made of other materials, for example, stainless steel). The mobile phase is made up of an eluent and the separation of the sample components depends on the affinity that its components have for both phases. Typically, the eluent passes through the column by gravity, although modern methods have been developed where it is driven by pumps that apply pressure.
Continue in: Chromatography