We explain what atomic models are and how they have evolved, from Antiquity to modern times.
What are atomic models?
The different models are known as atomic models. graphic representations of the structure and functioning of atoms. Atomic models have been developed throughout the history of humanity based on the ideas that were used in each era regarding the composition of matter.
The first atomic models date back to classical antiquity when philosophers and naturalists ventured to think and deduce the composition of the things that exist, that is, of matter.
See also: Octet Rule
Atomic model of Democritus (450 BC)
The “Atomic Theory of the Universe” was created by the Greek philosopher Democritus together with his mentor, Leucippus. At that time, knowledge was not achieved through experimentation, but through logical reasoning, based on the formulation and debate of ideas.
Democritus proposed that the world was made up of very small and indivisible particles of eternal existence, homogeneous and incompressible, whose only differences were in shape and size, never in internal functioning. These particles were baptized “atoms”, a word that comes from the Greek atémnein and means “indivisible.”
According to Democritus, the properties of matter were determined by the way atoms were grouped together. Later philosophers such as Epicurus added the random movement of atoms to the theory.
Dalton's atomic model (1803 AD)
The first atomic model with scientific bases was born within chemistry, proposed by John Dalton in his “Atomic Postulates”. He maintained that everything was made of atoms, indivisible and indestructible even through chemical reactions.
Dalton proposed that the atoms of the same chemical element were equal to each other and had the same mass and the same properties. On the other hand, he proposed the concept of relative atomic weight (the weight of each element with respect to the weight of hydrogen), comparing the masses of each element with the mass of hydrogen. He also proposed that atoms can combine with each other to form chemical compounds.
Dalton's theory had some flaws. He stated that chemical compounds were formed using the smallest possible number of atoms of their elements. For example, the water molecule, according to Dalton, would be HO and not H2Or, which is the correct formula. On the other hand, he said that elements in the gaseous state were always monatomic (composed of a single atom), which we know is not real.
Lewis atomic model (1902 AD)
Also called “Cubic Atom Model”, in this model Lewis proposed the structure of atoms distributed in the shape of a cube in whose eight vertices the electrons were found. This allowed progress in the study of atomic valences and chemical bonds, especially after its update by Irving Langmuir in 1919, where he proposed the “cubic octet atom.”
These studies were the basis of what is known today as the Lewis diagram, a very useful tool to explain covalent bonding.
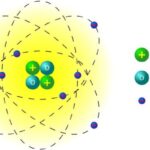
Thomson's atomic model (1904 AD)
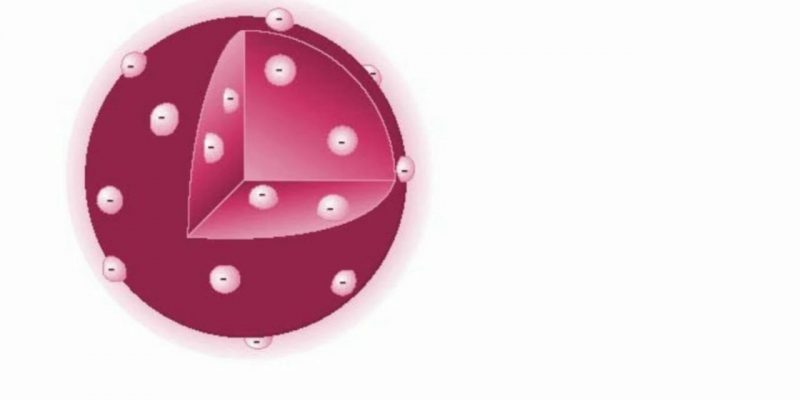
Proposed by JJ Thomson, discoverer of the electron in 1897, this model is prior to the discovery of protons and neutrons, so assumed that atoms were composed of a positively charged sphere and the negatively charged electrons were embedded in it, like raisins in pudding. This metaphor gave the model the epithet “Pruny Pudding Model.”
This model made an incorrect prediction of the positive charge in the atom, since it stated that it was distributed throughout the atom. This was later corrected in Rutherford's model where the atomic nucleus was defined.
Rutherford's atomic model (1911 AD)
Ernest Rutherford conducted a series of experiments in 1911 using gold foil. In these experiments he determined that the atom is composed of a positively charged atomic nucleus (where most of its mass is concentrated) and electrons, which rotate freely around this nucleus. In this model, the existence of the atomic nucleus is proposed for the first time.
Continue in: Rutherford's atomic model
Bohr's atomic model (1913 AD)
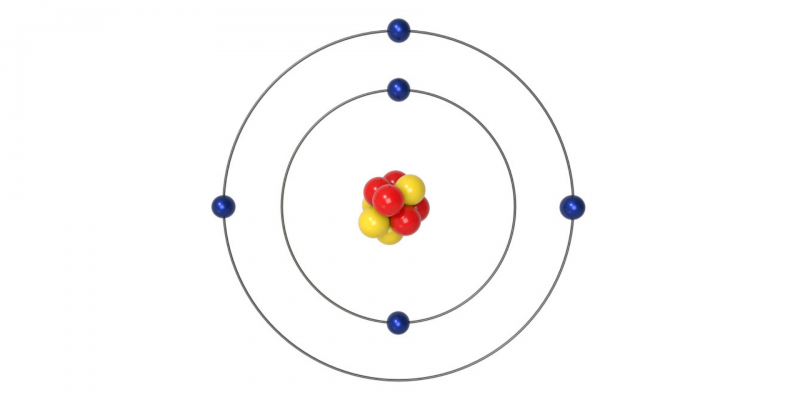
This model gives rise to quantum postulates in the world of physics, so It is considered a transition between classical and quantum mechanics. Danish physicist Niels Bohr proposed this model to explain how electrons could have stable orbits (or stable energy levels) surrounding the nucleus. It also explains why atoms have characteristic emission spectra.
In the spectra made for many atoms, it was observed that electrons of the same energy level had different energies. This showed that there were errors in the model and that energy sublevels must exist at each energy level.
Bohr's model is summarized in three postulates:
- Electrons travel in circular orbits around the nucleus without radiating energy.
- The orbits allowed for electrons are those with a certain value of angular momentum (L) (amount of rotation of an object) that is an integer multiple of the value, with h=6.6260664×10-34 yn=1, 2, 3….
- Electrons emit or absorb energy when jumping from one orbit to another and in doing so they emit a photon that represents the difference in energy between both orbits.
Sommerfeld's atomic model (1916 AD)
This model It was proposed by Arnold Sommerfield to try to cover the deficiencies that Bohr's model presented.
It was based on part of the relativistic postulates of Albert Einstein. Among its modifications is the statement that The orbits of the electrons were circular or elliptical that electrons had tiny electric currents and that starting from the second energy level there were two or more sublevels.
Schrödinger's atomic model (1926 AD)
Proposed by Erwin Schrödinger based on the studies of Bohr and Sommerfeld, He conceived of electrons as undulations of matter which allowed the subsequent formulation of a probabilistic interpretation of the wave function (magnitude that serves to describe the probability of finding a particle in space) by Max Born.
This means that the position of an electron or its momentum can be studied probabilistically, but not both at the same time, due to the Heisenberg Uncertainty Principle.
This is the atomic model in force at the beginning of the 21st century, with some later additions. It is known as the “Quantum-Wave Model”.
References
- “Fundamentals of Physics” by Halliday, David. 8th edition, Wiley (2007). ISBN 0-471-15950-6.