We explain what homogeneous mixtures are and what other types of mixtures exist. Examples of homogeneous type mixtures.

What is a homogeneous mixture?
A homogeneous mixture is a union of two or more substances in which the original substances cannot be distinguished. The elements that make up the homogeneous mixture cannot be distinguished with the naked eye, but they are physically separable since a chemical reaction does not take place between them. For example: Tea with sugar or a mixture of water and alcohol.
Mixtures are very commonly used and are used in aspects as diverse as cooking, construction, jewelry, among many others.
To achieve a mixture, it is enough to mechanically join two or more different substances until they form a joint matter. It is important to know that the components mix but retain their chemical properties and, generally, they can be separated again through procedures such as sieving, filtration, magnetic separation, decantation, centrifugation, among others.
a mix can be made with substances in liquid, solid or gaseous state. For example, alloys, which are homogeneous mixtures of metals. Another example of a homogeneous mixture is solutions in which, generally, at least one component is a liquid. For example: salt dissolved in water.
Types of homogeneous mixture
Mixtures can be of two types:
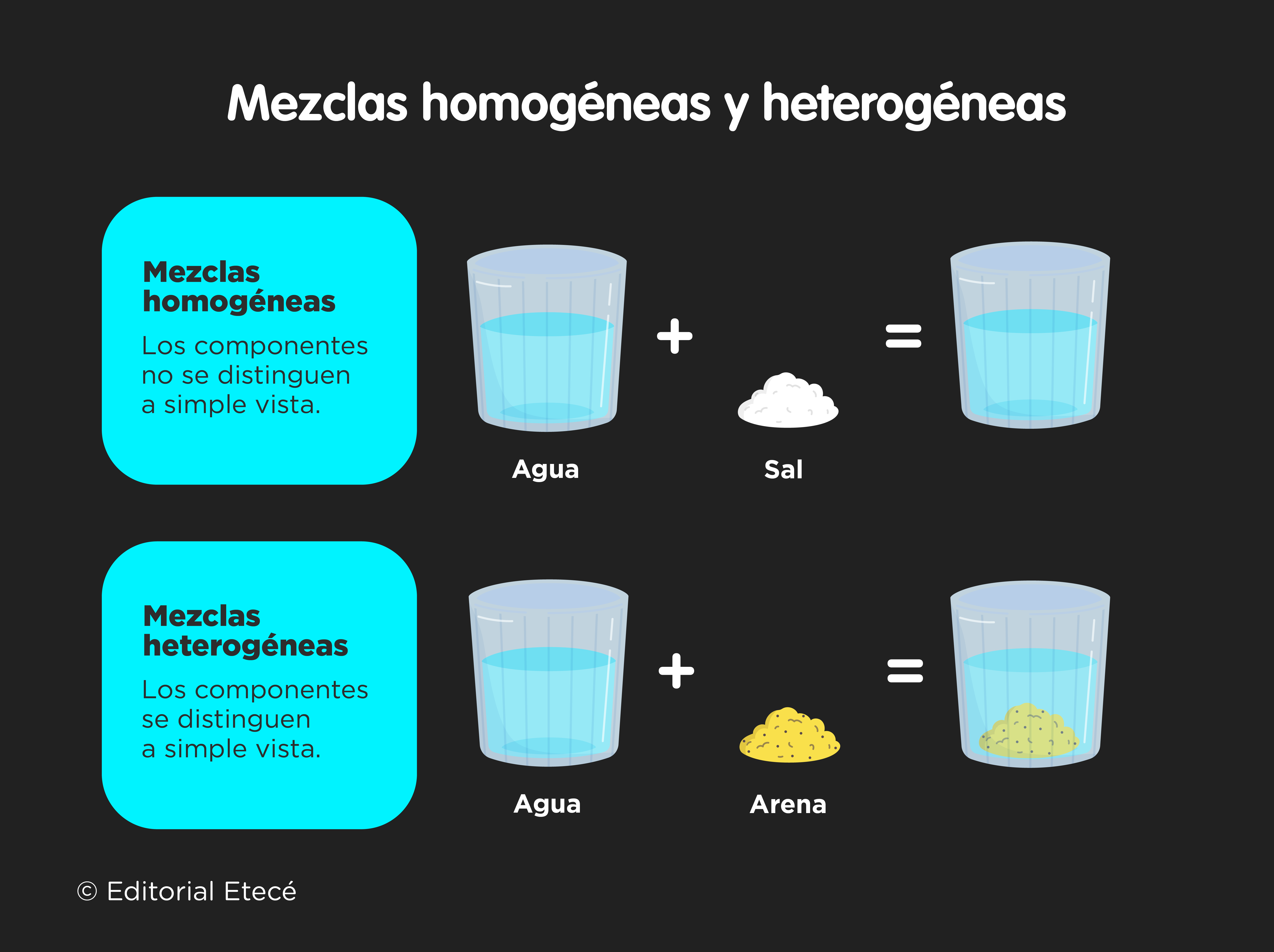
- Homogeneous mixtures. They are those mixtures in which each of the components that make it up cannot be differentiated with the naked eye. They are also known as solutions. For example: Powdered juices.
- heterogeneous mixtures. They are those mixtures in which the components that constitute them can be seen with the naked eye, given that they are usually distributed unevenly. Depending on the size of the substances, they can be thick mixtures (the particles are large in size) or suspensions (the particles are small in size). For example: Water with oil.
Examples of homogeneous mixtures
Some examples of homogeneous mixtures are:
- The air. It is the mass of gases that is attracted to the Earth's surface by gravity and is composed of: nitrogen, argon, oxygen, carbon dioxide, among others. These gases cannot be distinguished with the naked eye.
- Coffee with sugar. It is a homogeneous mixture because when you dissolve a spoonful of sugar in the coffee, the white color of this ingredient is no longer noticeable (although you can feel its flavor when you take a sip) and the mixture continues to have the dark color of coffee.
- salt water. Sea water is a homogeneous mixture that is composed of water, salts and other dissolved substances. However, when observing the water, these components cannot be differentiated, which are what give it salinity.
- chocolate milk. It is a drink that consists of a homogeneous mixture made up of milk and one or several tablespoons of chocolate powder. The mixture has a dark color and, if it does not have lumps, the components cannot be recognized with the naked eye.
- Steel. It is an alloy of iron, its base metal, with other metallic or non-metallic components such as carbon, nickel or copper (depending on the type of steel being sought). The result is a metal in which the components cannot be distinguished and that combines their physical properties. The same thing happens with other metal alloys such as bronze or white gold.
- Water and alcohol. It is a liquid and homogeneous mixture that is obtained by mixing water and some distilled alcohol. In this mixture, it cannot be seen with the naked eye how much of each component there is.
| Chlorinated water | Bronze (copper-tin alloy) | White gold (alloy of gold and another metal) |
| Olive oil | Coffee with milk | Perfume |
| sugar water | Chocolate | Shampoo |
| Water with baking soda | Mouthwash | Tea |
| Water with bleach | powdered juice | Tea with sweetener |
| Water with lemon | Ketchup | milk tea |
| Syrup | Brass (copper and zinc alloy) | Vaseline |
| Alpaca (copper, zinc and nickel alloy) | pizza dough | Glass |
| Bitumen | Honey | Came |
Continue with: Pure substance
References
- “Mixture” on Wikipedia.
- “Substances and mixtures” in Lumen.
- “Homogeneous and heterogeneous mixtures: what are they?” on ABC.
- “Mixtures of materials” in Editorial Kapelusz.





