We explain what density is and what types of density exist. Examples of the absolute density of different substances.

What is the density of matter?
The density is a scalar magnitude frequently used in physics and chemistry, which refers to the amount of mass present in a given body or substance per unit of volume. It is usually represented by the symbol ρ.
Two bodies or substances of the exact same size and proportions can have different densities, and this is measured through the average density, which is the relationship between the mass of a body and the volume it occupies in space, according to the following formula:
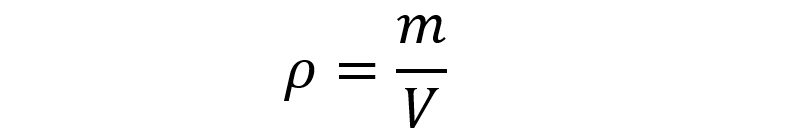
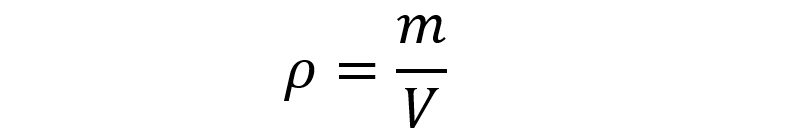
where m is the mass and V is the volume, so the unit of measurement of density in the International System will be the kilogram per cubic meter (kg/m3) or equivalent units of measurement.
Variations in temperature and pressure affect the density measurement of a substance.
The density of matter is often associated with the story of the Greek philosopher Archimedes, who was supposedly tasked with determining whether the king's crown had been forged using pure gold or whether it had been made from an alloy with other metals.
During an immersion bath, Archimedes realized that he could calculate the volume of the crown by submerging it in water and measuring the displacement of the liquid, without having to melt or break it. Then I could weigh the crown (to have its mass) and determine, using the formula detailed above, the density of the crown. He compared the values of the calculated density of the crown with the density of pure gold (which is a constant), and knew in this way whether it was pure gold or an alloy, since the density of gold would have varied when mixed with other gold. metals.
See also: Specific gravity
Types of density
There are several types of density of matter:
- Absolute density Generally, we speak of absolute density when we use the term densityand it is an intensive magnitude calculated, as we said above, from the volume and mass. Its SI units of measurement are (kg/m3).
- Relative density This other type, on the other hand, arises from the comparison between the density of the substance in question and some other that serves as a reference, so it is a dimensionless magnitude (without units). For liquids and solids, the density of water is used as a reference (at 1atm and 4 °C), while for gases the density of air is used (at 1atm and 0 °C). It is calculated in the following way:
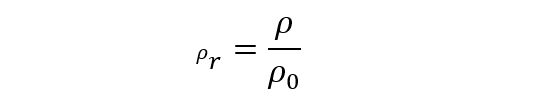
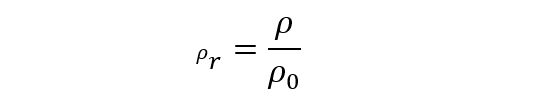
where 𝛒r is the relative density, 𝛒 is the absolute density and 𝛒0 is the density of the reference substance. - Apparent density It is applied to heterogeneous materials, as well as porous ones, whose mixture affects the density (being lower than if each element were compacted separately). Hence this type of density does not depend on the nature of the matter, but on the way it is arranged. For example, if we have a hydrated material, its apparent density will be:
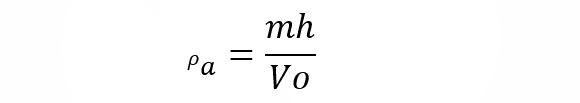
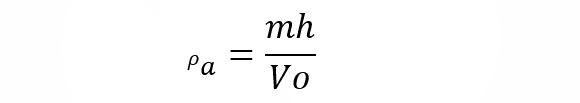
where 𝛒to is the apparent density of the material, mh is the mass of the dried material and V0 is the volume of the hydrated material, that is, without drying.
Examples of density
Some examples of the absolute density of different elements and substances (expressed in their appropriate units) can be:
- Magnesium (Mg) 1.738g/cm3
- Calcium (Ca). 1.54g/cm3
- Iron (Fe) 7.874g/cm3
- Molybdenum (Mo). 10.22g/cm3
- Silver (Ag). 10.5g/cm3
- Gold (Au) 19.3g/cm3
- Iridium (Ir). 22.562g/cm3
- Dubnium (Db) 29.3g/cm3
- Bohrio (Bh) 37.1g/cm3
- Water (H2O). 1g/cm3
- Oil 0.92g/cm3
- Air. 1.225kg/m3