We explain what the mole is and how this magnitude is calculated. Also, its general characteristics and what is its volume.

What is the mole?
The mole is one of the magnitudes stipulated by the International System of Units (SI). Its symbol is “mol”.
The mole is defined as the amount of matter that contains a certain number of elemental entities (atoms, molecules, etc.) equivalent to the number of atoms in 12 grams of the carbon-12 isotope (12C).
The mass of one mole of substance (called molar mass) is equivalent to the atomic or molecular mass (depending on whether a mole of atoms or molecules has been considered) expressed in grams.
Avogadro's number (NTO) is the number of particles (molecules, atoms, electrons) contained in one mole of any substance. It is a constant that corresponds to the value of 6.022×10^23 mol-1. Therefore, 1 mole of any substance contains 6.022×10^23 elemental entities of that substance. On the other hand, Avogadro's number allows conversions to be established between the gram and the atomic mass unit, with 6.022×10^23 amu (atomic mass unit) equal to 1 gram. Avogadro's number is the number of atoms contained in 1 mole of atoms whose mass is equal to the atomic mass of the element.
See also: Specific heat
How is the mole calculated?
To calculate the moles it is necessary to know the atomic or molecular mass depending on whether they are atoms or compounds respectively. So, to calculate the number of moles of molecules or atoms of any substance, the fraction must be made between the mass of the substance over its molecular or atomic mass. For example, if we want to calculate the number of moles of X we write:
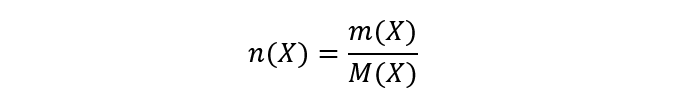
Where: is the number of moles of X, m(X) is the mass of X and M(X) is the atomic or molecular mass of X.
What is the volume of one mole?
When substances are in a gaseous state it is possible to calculate the volume occupied by one mole. Volume is a measure of the magnitude of the extension of a body and its unit is the cubic meter (m3) in the SI.
Under the standard conditions of temperature and pressure (T=0 ⁰C and P=1 atm), The volume of one mole of gas is equal to 22.4 liters (L). This value is called molar volume (Vm) and corresponds to the so-called ideal gases. Real gases have values of Vm slightly different from this value. For example, the CO2 has Vm=22.3L.