We explain what acetic acid is and the formula of this substance. In addition, its physical and chemical properties and its different uses.

What is acetic acid?
Acetic acid, also called methylcarboxylic acid or ethanoic acid, is an organic substance present in the composition of vinegar responsible for its typical sour smell and taste.
It is a weak acid frequent in various fermentation processes like those that occur in wine (when it is vinegared) or in certain fruits. It is commonly used in cooking, as a vegetable cleaner or salad dressing (like vinegar, that is, diluted with water to 3 to 5% solute). In pure proportions it can be risky for health.
See also: Sulfuric acid
Acetic acid formula
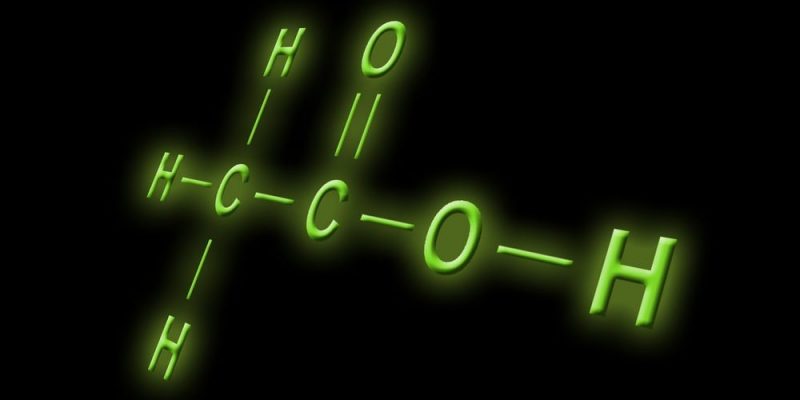
Acetic acid responds to the chemical formula c2h4EITHER2 and its semi-developed formula is CH3COOH.
Seen this way, it is nothing more than a methyl group (CH3-) with a carboxyl group (-COOH) linked by a single bond between its carbon atoms.
Physical properties of acetic acid
The appearance of acetic acid is crystalline, at least when it is found as its acetate ion (a salt or ester of the acid). It has a melting point of 16.6 ºC and a boiling point of 117.9 ºC, thanks to which It is possible to separate it from water by distillation. It also has a density of 1049 kg/m³ and a moderate acidity of 4.8 pKa.
It is a flammable and corrosive material at the same time which makes its handling delicate, as it is capable of seriously irritating the skin, eyes, and the digestive tract (due to ingestion) or respiratory tract (due to inhalation).
Chemical properties of acetic acid
Acetic acid belongs to carboxylic acids (characterized by the presence of a carboxyl functional chemical group: -COOH), and is usually placed in the classifications between formic or methanoic acid (which has a single carbon atom) and propanoic acid (which already has a chain of three carbon atoms).
It is a weak acid, common as a biological metabolite and as a substrate for acetyltransferase enzymes. It is usually obtained through four different methods:
- Carbonylation of methanol Reaction of methanol with carbon monoxide, using iodomethane and a catalyst.
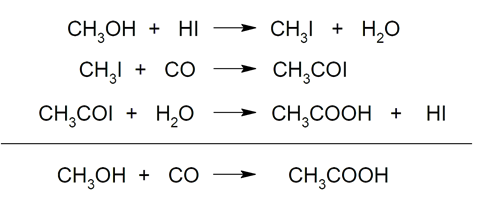
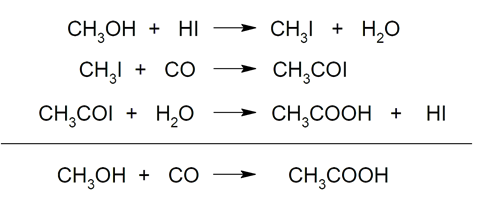
- Oxidation of acetaldehyde. Oxidation of acetaldehyde by oxygen using catalysts.
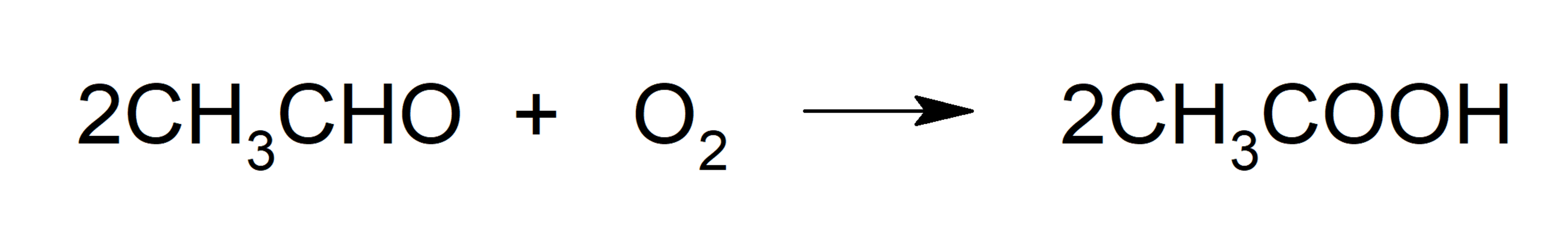
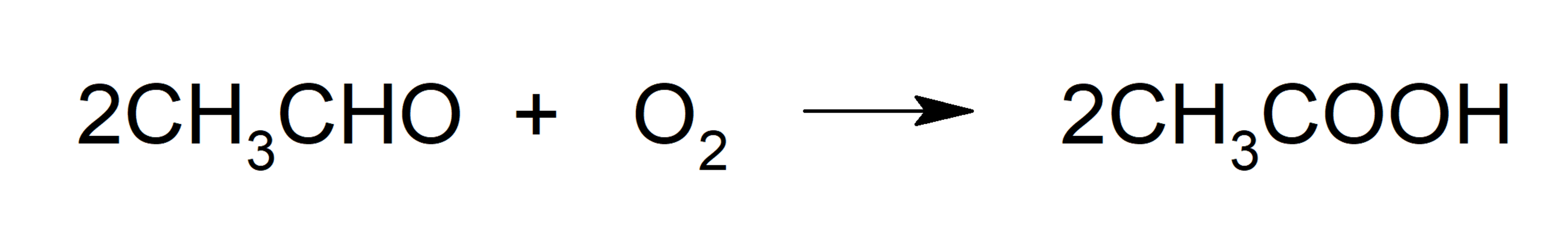
- Oxidative fermentation. Fermentation produced by bacteria of the genus acetobacter (bacteria that can convert ethanol into acetic acid in an air environment).
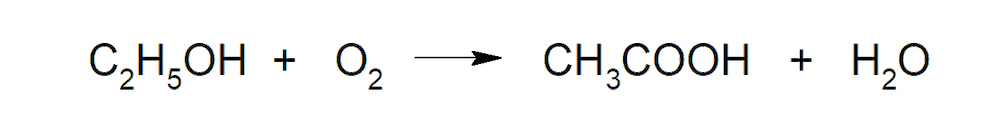
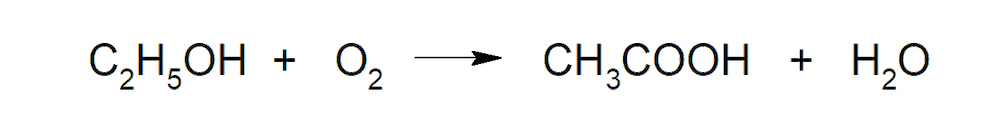
- Anaerobic fermentation. Some anaerobic bacteria (that do not use O2 to carry out their metabolism) produce acetic acid from sugars.
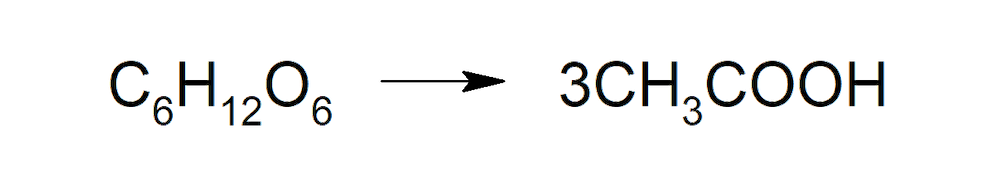
Applications and uses

Acetic acid has numerous applications in industry:
- Control of wax moths (galleriosis) in beekeeping.
- Important component (in salts or esters) for the manufacture of nylon, rayon, cellophane and other films.
- Component of fixative substances in the preservation of organic tissues in the laboratory.
- Part of the chemicals used in photographic development.
- Medical dye to reveal Human Papillomavirus (HPV) lesions.
- Component of general-purpose cleaners and stain removers.
- Culinary uses (vinegar).





