We explain what alcohols are, their classification, nomenclature and properties. Also, examples and importance in the industry.
What are alcohols?
Alcohols are true organic chemical compounds, which have one or more hydroxyl chemical groups in their structure (-OH) covalently bonded to a saturated carbon atom (that is, with single bonds only to adjacent atoms), forming a carbinol group (-C-OH).
Alcohols are organic compounds very common in nature which play important roles in living organisms, especially in organic synthesis.
Its name comes from Arabic al-kukhūlwhich literally translates as “spirit” or “distilled liquid.” This is because ancient Muslim alchemists called alcohols “spirit” and also perfected distillation methods in the 9th century. Later studies allowed us to know the chemical nature of these compounds, especially Lavoisier's contributions regarding the fermentation of brewer's yeast.
The alcohols They can be toxic and even lethal to the human body if ingested in high doses. Furthermore, when consumed by humans, they can act as depressants of the central nervous system, causing a state of drunkenness and causing more uninhibited behavior than normal.
On the other hand, alcohols have antibacterial and antiseptic properties that allow their use in the chemical industry and medicine.
Types of alcohols
Alcohols can be classified according to the number of hydroxyl groups they present in their structure:
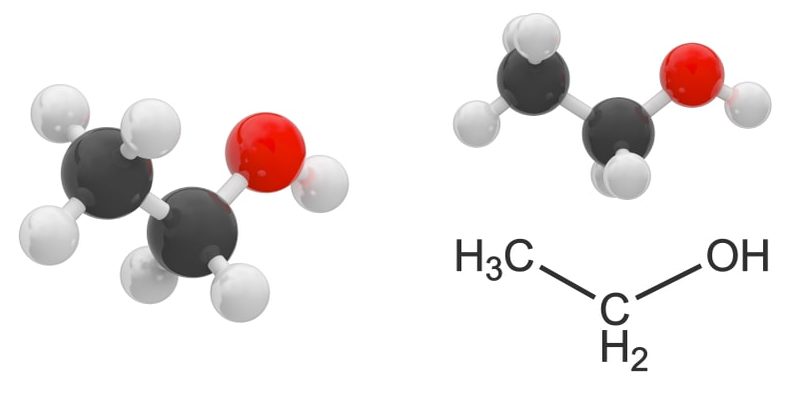
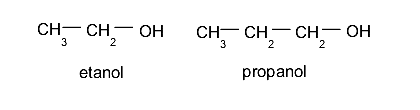
- Monoalcohols or alcohols. These contain a single hydroxyl group. For example:
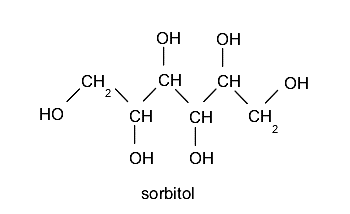
- Polyalcohols or polyols They contain more than one hydroxyl group. For example:
Another way to classify alcohols is according to the position of the carbon to which the hydroxyl group is bonded, also taking into account how many carbon atoms this carbon is also bonded to:
- Primary alcohols The hydroxyl group (-OH) is located on a carbon bonded to another single carbon atom. For example:
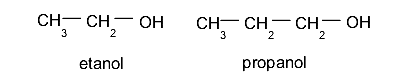
- Secondary alcohols The hydroxyl group (-OH) is located on a carbon linked to two other different carbon atoms. For example:
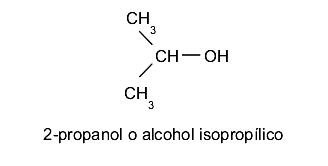
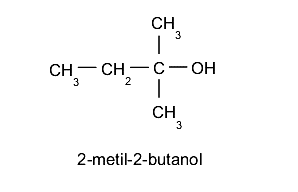
- Tertiary alcohols The hydroxyl group (-OH) is located on a carbon linked to three other different carbon atoms. For example:
Nomenclature of alcohols
Like other organic compounds, alcohols have different ways of being named, which we will explain below:
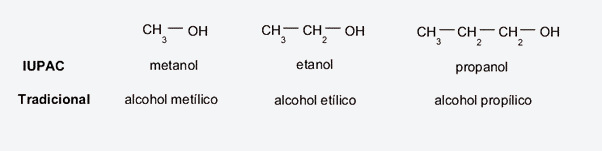
- Traditional method (non-systematic). Attention is paid, above all, to the carbon chain to which the hydroxyl (generally an alkane) adheres, to rescue the term with which it is named, prefixing the word “alcohol” and then adding the suffix -yl in instead of -ano. For example:
- If it is a methane chain, it will be called methyl alcohol.
- If it is an ethane chain, it will be called ethyl alcohol.
- If it is a propane chain, it will be called propyl alcohol.
- IUPAC method Like the previous method, attention will be paid to the precursor hydrocarbon, to rescue its name and simply add the ending -ol instead of -ane. For example:
- If it is a methane chain, it will be called methanol.
- If it is an ethane chain, it will be called ethanol.
- If it is a propane chain, it will be called propanol.
Eventually, it will be necessary to indicate in some way the location of the hydroxyl group in the chain, for which a number is used at the beginning of the name. It is important to keep in mind that the longest hydrocarbon chain is always chosen as the main chain and the position of the hydroxyl group should be selected using the least numbering possible. For example: 2-butanol.
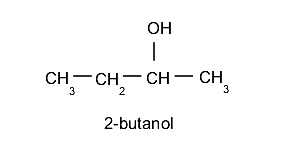
Physical properties of alcohols
Alcohols are generally colorless liquids that have a characteristic odor, although, in less abundance, they can also exist in a solid state. They are soluble in water since the hydroxyl group (-OH) has a certain similarity to the water molecule (H2O), which allows them to form hydrogen bonds. In this sense, the most water-soluble alcohols are those with the lowest molecular mass, that is, those with smaller and simpler structures. As the number of carbon atoms and the complexity of the carbon chain increases, the alcohols are less soluble in water.
The density of alcohols is greater according to the increase in the number of carbon atoms and the branches of its hydrocarbon chain. On the other hand, the formation of hydrogen bonds not only influences solubility, but also its melting and boiling points. The larger the hydrocarbon chain, the more hydroxyl groups it has and the more branches it has, the higher the values of these two properties will be.
Chemical properties of alcohols
The alcohols have a dipolar character similar to that of water, due to its hydroxyl group. This makes them polar substances (with a positive and a negative pole).
Because of this, alcohols can behave like acids or as bases depending on what reagent they react with. For example, if an alcohol is reacted with a strong base, the hydroxyl group is deprotonated and the oxygen retains its negative charge, acting as an acid.
On the contrary, if an alcohol is confronted with a very strong acid, the electron pairs of the oxygen cause the hydroxyl group to become protonated, acquire a positive charge and behave like a weak base.
On the other hand, alcohols can participate in the following chemical reactions:
- Halogenation. Alcohols react with hydrogen halides to give alkyl halides and water. Tertiary alcohols react more easily than primary and secondary alcohols. Some examples of these reactions are:
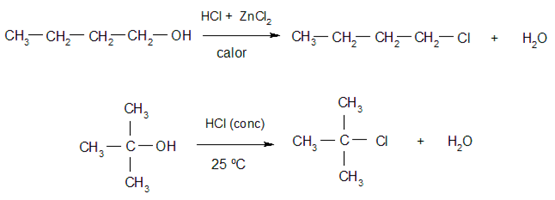
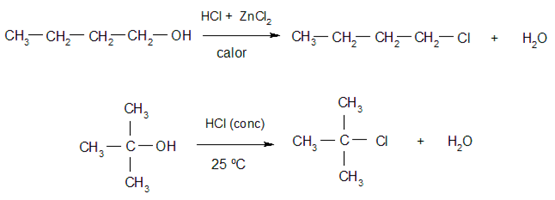
- Oxidation. Alcohols are oxidized by reacting with certain oxidizing compounds, forming different products depending on the type of alcohol that is oxidized (primary, secondary or tertiary). For example:
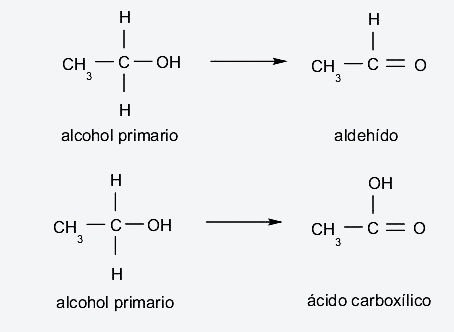
- Primary alcohols They occur if, when oxidized, they lose a hydrogen atom that is bonded to carbon, which in turn is bonded to the hydroxyl group, forming aldehydes. On the other hand, if they lose the two hydrogen atoms of this carbon, they form carboxylic acids.

- Secondary alcohols When oxidized, they lose the only hydrogen atom bonded to carbon that has the hydroxyl group and form ketones.
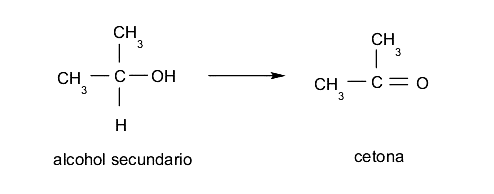

- Tertiary alcohols They are resistant to oxidation, that is, they do not rust unless very specific conditions are imposed on them.
- Primary alcohols They occur if, when oxidized, they lose a hydrogen atom that is bonded to carbon, which in turn is bonded to the hydroxyl group, forming aldehydes. On the other hand, if they lose the two hydrogen atoms of this carbon, they form carboxylic acids.
- Dehydrogenation Alcohols (only primary and secondary) when subjected to high temperatures and in the presence of certain catalysts, lose hydrogen to form aldehydes and ketones.
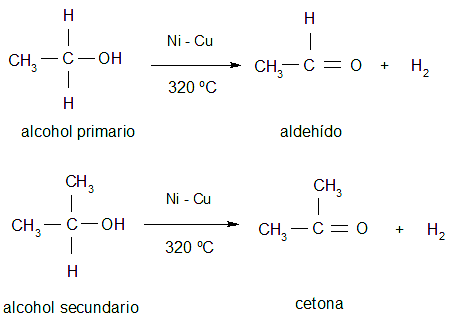

- Dehydration. It consists of adding a mineral acid to an alcohol to extract the hydroxyl group and obtain the corresponding alkene through elimination processes.
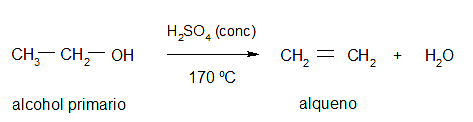
Importance of alcohols

Alcohols are substances of great chemical value. As raw material, are used to obtain other organic compounds in laboratories. Also as a component of industrial products for everyday use, such as disinfectants, cleaners, solvents, perfume bases.
They are also used in the manufacture of fuels, especially in the biofuels industry, an alternative to those of fossil origin. It is common to see them in hospitals, first aid kits or similar.
On the other hand, certain alcohols are for human consumption (especially ethanol), part of numerous spirit drinks in different degrees of refinement and intensity.
Examples of alcohols
Some examples of alcohols widely used on a daily basis are:
- methanol or methyl alcohol (CH3OH)
- ethanol or ethyl alcohol (C2h5OH)
- 1-propanol, propanol or propyl alcohol (C3h7OH)
- isobutanol (C4h9OH)
Continue with: Biomolecules
References
- “Alcohols” https://es.wikipedia.org/
- “Study of some properties of alcohols” at the Autonomous University of the State of Hidalgo. https://www.uaeh.edu.mx/
- “Organic nomenclature: alcohols” (video) in Quimiayudas. https://www.youtube.com/
- “Alcohols” (video) https://es.khanacademy.org/
- “Alcohol” https://www.sciencedaily.com/
- “Alcohols” https://courses.lumenlearning.com/
- “Alcohol (chemical compound)” https://www.britannica.com/