We explain what the atomic number is and its relationship with the atomic mass. Also, the atomic number of each chemical element.
What is atomic number?
In both physics and chemistry, the atomic number is the total number of protons that make up the atomic nucleus of a chemical element certain.
It is usually denoted by the letter Z (from the German word zahl“number”) and placed as a subscript to the left of the chemical symbol of the element in question, just below the mass number A (number of nucleons in the nucleus, that is, sum of the number of protons and the number of neutrons). For example:
2311Na (element: sodium, atomic number: 11, and mass number: 23).
All atoms are made up of subatomic particles: some are part of its nucleus (protons and neutrons) and others revolve around it (electrons). Protons have a positive charge, neutrons have a neutral charge, and electrons have a negative charge (electrons).
Given that Atoms in nature are electrically neutral the number of positive and negative particles is the same, so if an atom has Z = 11, it will have eleven protons and eleven electrons around it.
Furthermore, the atomic number allows you to organize the known elements in the Periodic Table go from the lowest to the highest number of protons in the nucleus as you move through the rows and columns of the table. For example, hydrogen (H) has only one proton (Z = 1), while oganeson (Og) has one hundred and eighteen (Z = 118). This way you can differentiate light elements from heavy elements.
See also: Atomic models
Examples of atomic number
These are the atomic numbers of the complete Periodic Table:
| Hydrogen (H): Z = 1 | Helium (He): Z = 2 |
| Lithium (Li): Z = 3 | Beryllium (Be): Z = 4 |
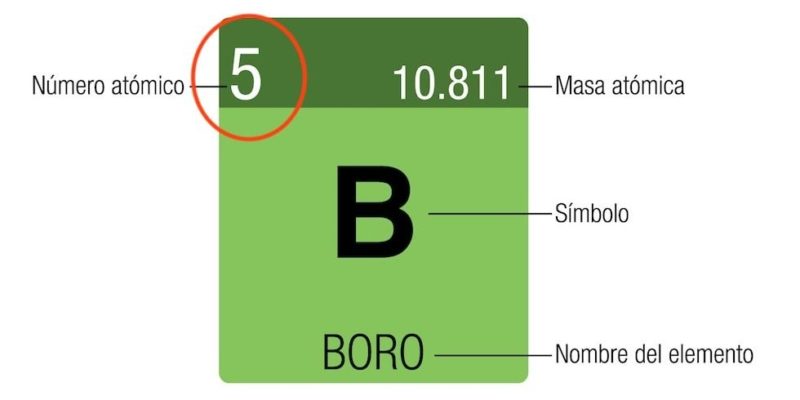
| Boron (B): Z = 5 | Carbon (C): Z = 6 |
| Nitrogen (N): Z = 7 | Oxygen (O): Z = 8 |
| Fluorine (F): Z = 9 | Neon (Ne): Z = 10 |
| Sodium (Na): Z = 11 | Magnesium (Mg): Z = 12 |
| Aluminum (Al): Z = 13 | Silicon (Si): Z = 14 |
| Phosphorus (P): Z = 15 | Sulfur (S): Z = 16 |
| Chlorine (Cl): Z = 17 | Argon (Ar): Z = 18 |
| Potassium (K): Z = 19 | Calcium (Ca): Z = 20 |
| Scandium (Sc): Z = 21 | Titanium (Ti): Z = 22 |
| Vanadium (V): Z = 23 | Chromium (Cr): Z = 24 |
| Manganese (Mn): Z = 25 | Iron (Fe): Z = 26 |
| Cobalt (Co): Z = 27 | Nickel (Ni): Z = 28 |
| Copper (Cu): Z = 29 | Zinc (Zn): Z = 30 |
| Gallium (Ga): Z = 31 | Germanium (Ge): Z = 32 |
| Arsenic (As): Z = 33 | Selenium (Se): Z = 34 |
| Bromine (Br): Z = 35 | Krypton (Kr): Z = 36 |
| Rubidium (Rb): Z = 37 | Strontium (Sr): Z = 38 |
| Yttrium (Y): Z = 39 | Zirconium (Zr): Z = 40 |
| Niobium (Ni): Z = 41 | Molybdenum (Mb): Z = 42 |
| Technetium (Tc): Z = 43 | Ruthenium (Ru): Z = 44 |
| Rhodium (Rh): Z = 45 | Palladium (Pd): Z = 46 |
| Silver (Ag): Z = 47 | Cadmium (Cd): Z = 48 |
| Indium (In): Z = 49 | Tin (Sn): Z = 50 |
| Antimony (Sb): Z = 51 | Tellurium (Te): Z = 52 |
| Iodine (I): Z = 53 | Xenon (Xe): Z = 54 |
| Cesium (Cs): Z = 55 | Barium (Ba): Z = 56 |
| Lanthanum (La): Z = 57 | Cerium (Ce): Z = 58 |
| Praseodymium (Pr): Z = 59 | Neodymium (Nd): Z = 60 |
| Promethium (Pr): Z = 61 | Samarium (Sm): Z = 62 |
| Europium (Eu): Z = 63 | Gadolinium (Gd): Z = 64 |
| Terbium (Tb): Z = 65 | Dysprosium (Dy): Z = 66 |
| Holmium (Ho): Z = 67 | Erbium (Er): Z = 68 |
| Thulium (Tm): Z = 69 | Ytterbium (Yb): Z = 70 |
| Lutetium (Lu): Z = 71 | Hafnium (Hf): Z = 72 |
| Tantalum (Ta): Z = 73 | Wolfram (W): Z = 74 |
| Rhenium (Re): Z = 75 | Osmium (Os): Z = 76 |
| Iridium (Ir): Z = 77 | Platinum (Pt): Z = 78 |
| Gold (Au): Z = 79 | Mercury (Hg): Z = 80 |
| Thallium (Tl): Z = 81 | Lead (Pb): Z = 82 |
| Bismuth (Bi): Z = 83 | Polonium (Po): Z = 84 |
| Astatine (At): Z = 85 | Radon (Rn): Z = 86 |
| Francium (Fr): Z = 87 | Radius (Ra): Z = 88 |
| Actinium (Ac): Z = 89 | Thorium (Th): Z = 90 |
| Proactinium (Pa): Z = 91 | Uranium (U): Z = 92 |
| Neptunium (Np): Z = 93 | Plutonium (Pu): Z = 94 |
| Americium (Am): Z = 95 | Curie (Cm): Z = 96 |
| Berkelium (Bk): Z = 97 | Californium (Cf): Z = 98 |
| Einsteinium (Es): Z = 99 | Fermium (Fm): Z = 100 |
| Mendelevium (Md): Z = 101 | Nobelium (No): Z = 102 |
| Laurencio (Lr): Z = 103 | Rutherfordium (Rf): Z = 104 |
| Dubnium (Db): Z = 105 | Seaborgium (Sg): Z = 106 |
| Bohrio (Bh): Z = 107 | Hasium (Hs): Z = 108 |
| Meitnerium (Mt): Z = 109 | Darmstatio (Ds): Z = 110 |
| Roentgenium (Rg): Z = 111 | Copernicius (Cn): Z = 112 |
| Nihomium (Nh): Z = 113 | Flerovium (Fl): Z = 114 |
| Moscovium (Mc): Z = 115 | Livermorium (Lv): Z = 116 |
| Teneso (Ts): Z = 117 | Oganeson (Og): Z = 118 |
Mass number and atomic mass
The mass number is the sum of the protons and neutrons. It is denoted by the letter A (from the German Atomgewicht) as a superscript to the left of the chemical symbol (for example: 23Na).
The mass number It is usually approximately twice the atomic number since neutrons provide stability to the atomic nucleus, and thus overcome the natural repulsion between positively charged protons. Unlike the atomic number, the mass number varies for each isotope.
The mass number can be calculated according to the formula:
Mass number (A) = atomic number (Z) + number of neutrons (N).
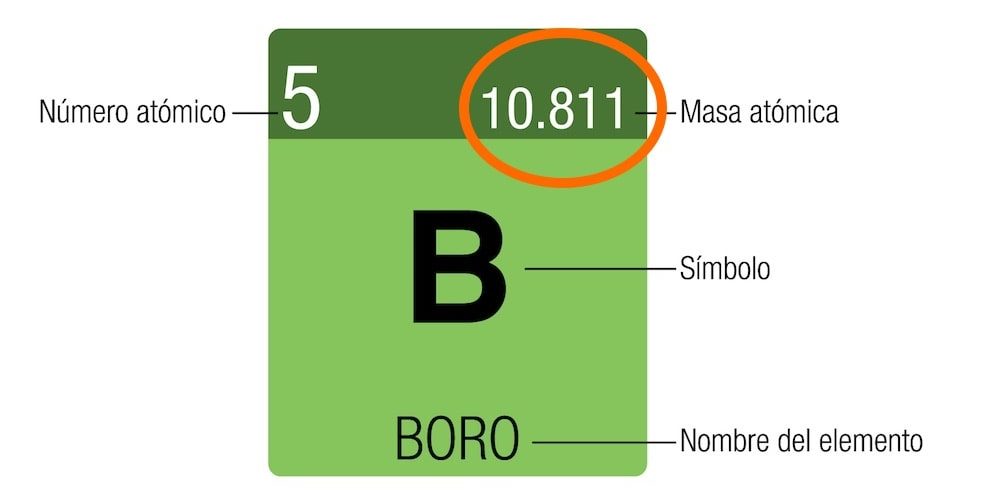
The mass number should not be confused with the atomic mass. Atomic mass is measured in amu units. (atomic mass unit) or Give (dalton). This unit is calculated from the carbon atom and each amu is one twelfth of its mass. The atomic mass of the most stable isotope appears on the periodic table.
Continue with: Valencia in chemistry
References
- “Atomic number” in Wikipedia.
- “Atomic number, mass number and isotopes” in Khan Academy.
- “What is the atomic number?” on Clickmica, questions and answers about chemistry.
- “Understanding Atomic Number and Atomic Mass” (video) in Ricochet Science.
- “Atomic number” in The Encyclopaedia Britannica.