We explain what a chemical element is, its characteristics and examples. Also, the periodic table and chemical compounds.

What is a chemical element?
A chemical element is each of the fundamental forms of matter. It always appears as atoms of the same and unique type and, therefore, cannot be decomposed into simpler substances using chemical reactions.
When we talk about a chemical element or simply an element, we refer to a certain type of known atoms which are distinguished from others in their nature and fundamental properties. This is usually expressed through different symbols for each one.
Chemical elements are atoms. But the following must be understood: atoms that are part of the same chemical element have the same number of protons in their nucleus (atomic number), even if they have different atomic masses.
There are atoms that have the same atomic number that is, they belong to the same chemical element, but have a different number of neutrons, so their mass number (sum of protons and neutrons) is different. These types of atoms are called isotopes.
In other words, each chemical element has different amounts of the isotopes that make it up.
For example, The element hydrogen has three natural isotopes: the protium (1H), deuterium (2H) and tritium (3H). Protium is composed of a proton and an electron (it has no neutrons), and is the most abundant isotope of hydrogen. Deuterium is made up of a proton and a neutron in its nucleus, and an electron that orbits it. The nucleus of tritium is made up of 1 proton and two neutrons, and is a radioactive isotope. Other isotopes of the element hydrogen have also been synthesized in laboratories.
When a chemical reaction occurs between two or more substances, it is their chemical elements that are exchanged and combined, constituting new atomic bonds and thus forming new forms of matter. Everything that exists is made up of combinations of the same elements.
See also: Valencia in chemistry
Chemical elements in the Periodic Table
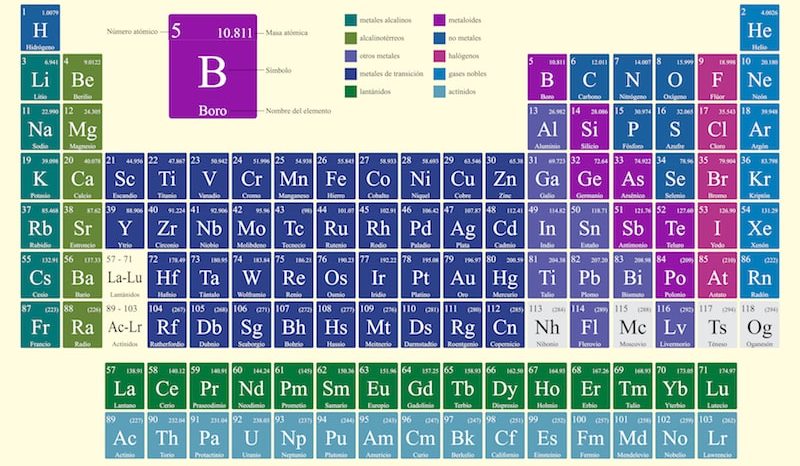
The Periodic Table of the Elements is a way to orderly represent all the known chemical elements (expressed through their chemical symbols). They are grouped based on their electronic and chemical properties, going from those with the lowest atomic number to those with the highest atomic number through their rows and columns.
This table It was presented in its first version by Dmitri Mendeleev in 1869. Since then it has been expanded, updated and improved, until obtaining its most current versions.
The Periodic Table distributes the elements in rows (called periods) and in columns (called groups) thus forming sets of elements classified into different categories, such as: metals (divided into alkaline, alkaline-earth, lanthanides, actinides, transition metals and other metals), metalloids and non-metals (divided into halogens, noble gases and other non-metals).
The IUPAC version of the Periodic Table can be seen here.
Examples of chemical elements

Some of the best known chemical elements are:
- Hydrogen (H)
- Carbon (C)
- Oxygen (O)
- Nitrogen (N)
- Phosphorus (P)
- Sulfur (S)
- Aluminum (Al)
- Iron (Fe)
- Chlorine (Cl)
- Iodine (I)
- Sodium (Na)
- Calcium (Ca)
- Potassium (K)
- Mercury (Hg)
- Silver (Ag)
- Gold (Au)
- Copper (Cu)
- Uranium (U)
- Argon (Ar)
- Zinc (Zn)
- Helium (He)
- Neon (Ne)
- Lead (Pb)
How many elements are there?
At the moment 118 different elements are known each described in the Periodic Table. However, some of them are synthetic, that is, artificial: they do not exist in nature but only in the laboratories of humanity.
The latest chemical technologies have made it possible to find up to 129 different elements many of which do not exist beyond a short period of time under very specific conditions in a specialized laboratory.
chemical compound
Chemical compounds are understood to the forms of matter that arise from the combination of different chemical elements. These can be relatively simple compounds such as some binary molecules (for example carbon dioxide (CO2)) or complex compounds with many different atoms, such as organic macromolecules (for example, DNA).
The truth is that all compounds They are substances that can be decomposed given the appropriate chemical reactions, into its constituent elements, becoming increasingly simpler until reaching monatomic or elemental substances. For example, water (H2O) it can be decomposed by electrolysis into hydrogen and oxygen molecules, both in gas form.
References
- “Chemical element” in Wikipedia.
- “Chemical elements arranged alphabetically” in LennTech.
- “Chemistry Tutorial: Chemical Elements” (video) at AtomicSchool.
- “Periodic Table of Elements” in International Union of Pure and Applied Chemistry (IUPAC).
- “Chemical element” in The Encyclopaedia Britannica.