We explain what a chemical emulsion is, what its phases are, how it is classified and what examples we find in everyday life.
What is an emulsion?
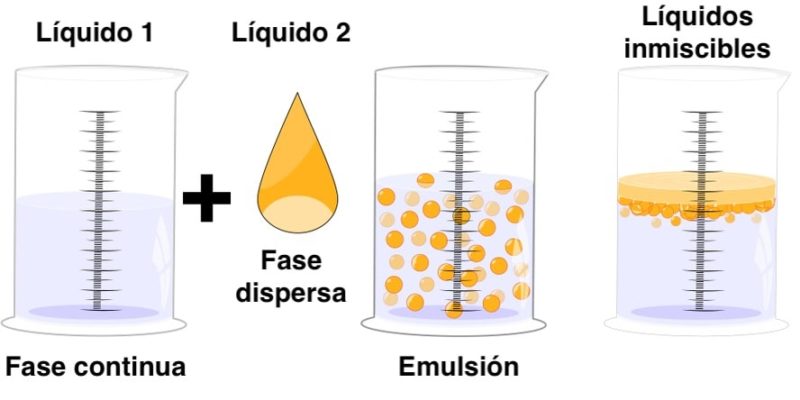
It is understood by chemical emulsion or simply emulsion the more or less homogeneous union of two immiscible liquids that is, they do not completely mix with each other. Emulsions consist of the dispersion of one liquid in another, both in different liquid phases.
They form what is commonly known as a colloid. Although these two terms are used interchangeably, emulsions differ from others colloids because they are always composed of liquid phases.
These two phases that make up an emulsion are always different and are classified as:
- Continuous phase The phase that is predominant to the other, that is, the one within which one of the liquids that make up the emulsion is dispersed. It is also called the “dispersing phase.”
- Dispersed phase The phase that is a minority compared to the other, that is, that disperses within the dispersing phase.
Due to different chemical and physical phenomena, emulsions always tend towards white, unless they are diluted emulsions (then tending towards blue) or concentrated (tending towards yellow). One and the other are distinguished based on the concentration gradient of one phase in the other.
Many times an emulsion It is formed due to the presence of emulsifying substances that is, particles that facilitate or promote the formation of emulsions between substances that, ordinarily, would find it much more difficult to do so.
In the same way, An emulsifier or emulsifier is a substance that stabilizes this type of mixtures preventing its phases from dispersing, acting as a binding material.
Emulsions can be of different types:
- Direct emulsions Emulsions that combine a lipophilic dispersed phase (attracted to fats) and a hydrophilic continuous phase (attracted to water).
- Reverse emulsions Emulsions that combine a hydrophilic dispersed phase and a lipophilic continuous phase, that is, the opposite of direct ones.
- Multiple emulsions Emulsions that present an inverse emulsion as a dispersed phase and an aqueous liquid as a continuous phase.
Examples of chemical emulsions

Some common and everyday emulsions are:
- The milk Water and fat emulsion
- The mayonnaise Water and oil emulsion.
- The vinaigrette sauce Oil and vinegar emulsion.
- The oil. Hydrocarbon emulsion.
- The ice cream Almost frozen milk and water emulsion.
- The butter Milk and oil emulsion.
- Bitumen or asphalt Hydrocarbon emulsion.
Continue with: Methods for separating mixtures
References
- “Emulsion” on Wikipedia.
- “Types of emulsions” in Technical Association of Bituminous Emulsions (Spain).
- “Emulsion” in Química.es.
- “Emulsion” in The Encyclopaedia Britannica.





