We explain what a chemical formula is, the types that exist, examples and its parts. Also, the symbols and chemical elements.
What is a chemical formula?
A chemical formula is a graphic expression of the elements that make up any chemical compound. The formulas express the numbers and proportions of their respective atoms and, in many cases, also the type of chemical bonds that join them. Each known molecule and/or compound has a chemical formula, as well as a name based on it according to the rules of chemical nomenclature.
There are various types of chemical formulas, each focused on a certain type of information, but in general terms all serve to understand the chemical nature of substances and to express what happens during a specific chemical reaction, in which some elements or compounds are transformed into others. For this reason, chemical formulas respond to a conventional system of representation of elements and molecules, that is, to a specialized technical language.
chemical formulas use the chemical symbols of the elements and logical proportions between them, expressed through mathematical symbols.
See also: Inorganic compound
Types of chemical formula
There are different types of chemical formula, useful to provide different information.
- Molecular formula It is a fairly basic type of formula that expresses the type of atoms present in a covalent compound and the amount of each one. It uses a linear sequence of chemical element symbols and numbers (as subscripts). For example, the molecular formula of glucose is C6h12EITHER6 (six carbon atoms, twelve hydrogen atoms and six oxygen atoms).
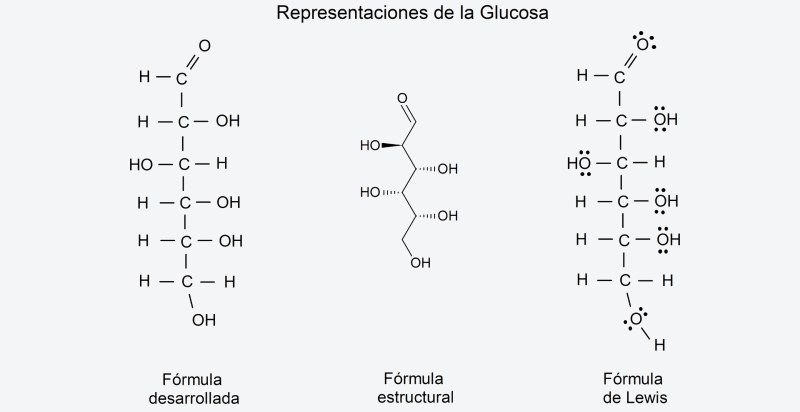
- Semi-developed formula. Similar to the molecular formula, it is a type of formula that expresses the atoms that make up the compound and also expresses the chemical bonds (lines) and their type (single, double, triple) between each atom of the compound. Carbon-hydrogen bonds are not represented in this formula. This is useful to identify the radical groups that make it up, as well as its chemical structure. For example, the semi-developed formula of glucose is, CH2OH – CHOH – CHOH – CHOH – CHOH – CHO.
- Developed formula. The developed formula is the next step in complexity from the semi-developed one. This representation indicates the bond and location of each atom of the compound within its respective molecules, in a Cartesian plane, representing the entire structure of the compound.
- Structural formula. To represent molecules not only in their structure and organization but also in their spatial form, an even more complex formula is needed, which uses two- or three-dimensional perspectives.
- Lewis formula Also called “Lewis diagrams” or “Lewis structures”, it is a representation similar to the developed formula of a compound, but which indicates the respective electrons shared in each chemical bond between atoms, according to the valence of the elements. involved. These electrons are represented by points linked with a line where there is a bond. Unshared electrons are also represented using dots on the corresponding atom. They are very specific formulas and for technical use.
Examples of chemical formula
Some examples of chemical (molecular) formulas of known compounds are:
- Oxygen EITHER2
- Ozone. EITHER3
- Carbon dioxide. CO2
- Carbon monoxide CO
- Water. h2EITHER
- Ammonia N.H.3
- Methane CH4
- Propane c3h8
- Sulfuric acid. h2SW4
- Hydrochloric acid HCl
- sodium chloride NaCl
- Baking soda NaHCO3
- Formaldehyde CH2EITHER
- Benzene c6h6
- Saccharose c12h22EITHER11
- Lime CaO
- Ethyl alcohol c2h5OH
- Monosodium glutamate c5h8NNaO4
- Penicillin c16h18N2EITHER4Yes
Parts of a chemical formula
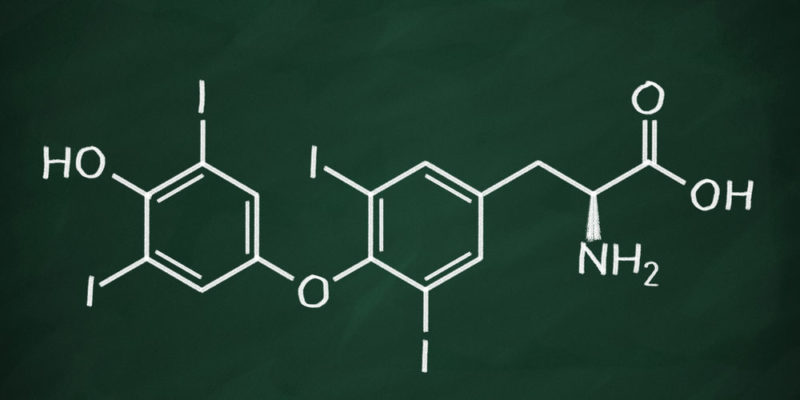
chemical formulas are made up of chemical symbols (letters) and subscripts (numbers) which express the type of atoms present in the substance and their quantity. However, in certain fields of chemistry (such as organic chemistry) compounds show certain structural and functional recurrence, which allows fragments of the molecule to be identified. These fragments are called “radicals” (molecular units with free electrons) or “functional groups” (atoms or molecular units responsible for the substance reacting in a certain way).
Examples of functional groups are: hydroxyl (-OH), carbonyl (=C=O), carboxyl (-COOH), among others.
Examples of radicals are: methyl (-CH3), ethyl (CH3CH2-), among others.
Chemical symbols
The chemical symbols are the minimum parts that make up any chemical formula and they represent each of the various chemical elements known to humanity, that is, the different types of atoms of which known matter is composed.
Each chemical element has a particular chemical symbol (usually derived from its historical Latin name).
Some examples of chemical symbols are:
- Carbon c
- Oxygen EITHER
- Phosphorus Q
- Hydrogen h
- Nitrogen N
- Iodine Yo
- Iron Faith
- Lead. Pb
- Aluminum To the
- Selenium HE
- Plutonium Pu
chemical elements

The chemical elements are the different types of atoms that make up matter and which are distinguished from each other according to the particular configuration of their subatomic particles (protons, neutrons and electrons).
The elements can be grouped according to their chemical properties that is, to the forces to which they respond more or less easily, to the behavior they exhibit in certain reactions, or to other structural features.
An example that illustrates well the definition of a chemical element is the following: isotopes 12C, 13C and 14C are some of the isotopes of the chemical element carbon (C).
The chemical elements are represented, classified and organized in the Periodic Table of the elements.
References
- “Chemical formula” https://es.wikipedia.org/
- “Chemical formulas” https://quimica.laguia2000.com/
- “Meaning of chemical formulas” in the Government of Aragon. http://aula.educa.aragon.es/
- “Empirical and molecular formula” http://bioprofe.com/
- “Chemistry Lesson: Chemical Formulas” (video) at GetChemistryHelp. https://www.youtube.com
- “Chemical Formula” https://www.britannica.com/