We explain what a chemical reaction is, the types that exist, its speed and other characteristics. Also, physical and chemical changes.

What is a chemical reaction?
Chemical reactions (also called chemical changes or chemical phenomena) are thermodynamic processes of transformation of the subject. These reactions involve two or more substances (reactants or reagents), which change significantly in the process, and can consume or release energy to generate two or more substances called products.
Every chemical reaction subjects matter to a chemical transformation, altering its structure and molecular composition (unlike physical changes that only affect its form or state of aggregation). chemical changes generally produce new substances different from the ones we had at the beginning.
Chemical reactions can occur spontaneously in nature (without human intervention), or they can also be generated by humans in a laboratory under controlled conditions.
Many of the materials we use daily are obtained industrially from simpler substances combined through one or several chemical reactions.
See also: Chemical compound
Physical and chemical changes in matter
Physical changes of matter are those that alter its form without changing its composition that is, without modifying the type of substance in question.
These changes have to do with changes in the state of aggregation of matter (solid, liquid, gaseous) and other physical properties (color, density, magnetism, etc.).
Physical changes are usually reversible since they alter the form or state of matter, but not its composition. For example, by boiling water we can convert a liquid into a gas, but the resulting vapor is still composed of water molecules. If we freeze water, it becomes a solid state but it remains chemically the same substance.
Chemical changes alter the distribution and bonds of atoms of the matter achieving that they combine in a different way, thus obtaining substances different from the initial ones, although always in the same proportion, since matter cannot be created or destroyed, only transformed.
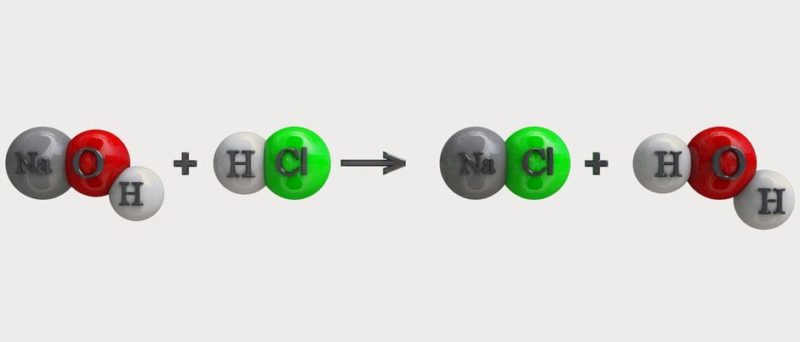
For example, if we react water (H2O) and potassium (K), we will obtain two new substances: potassium hydroxide (KOH) and hydrogen (H2). This is a reaction that normally releases a lot of energy and is therefore very dangerous.
Characteristics of a chemical reaction
Chemical reactions are generally irreversible processes, that is, involve the formation or destruction of chemical bonds between the reactant molecules, generating a loss or gain of energy.
In a chemical reaction, matter is profoundly transformed, although sometimes this recomposition cannot be seen with the naked eye. Even so, the proportions of the reactants can be measured, which is what stoichiometry deals with.
On the other hand, chemical reactions generate certain products depending on the nature of the reactants, but also on the conditions under which the reaction occurs.
Another important issue in chemical reactions is the speed at which they occur, since controlling their speed is essential for their use in industry, medicine, etc. In this sense, there are methods to increase or decrease the speed of a chemical reaction.
An example is the use of catalysts, substances that increase the speed of chemical reactions. These substances do not intervene in the reactions, they only control the speed at which they occur. There are also substances called inhibitors, which are used in the same way but cause the opposite effect, that is, they slow down the reactions.
How is a chemical reaction represented?
chemical reactions are represented by chemical equations that is, formulas in which the participating reactants and the products obtained are described, often indicating certain conditions of the reaction, such as the presence of heat, catalysts, light, etc.
The first chemical equation in history was written in 1615 by Jean Begin in one of the first treatises on chemistry, the Tyrocinium Chymicum. Today they are commonly taught and thanks to them we can more easily visualize what is happening in a given reaction.
The general way to represent a chemical equation is:
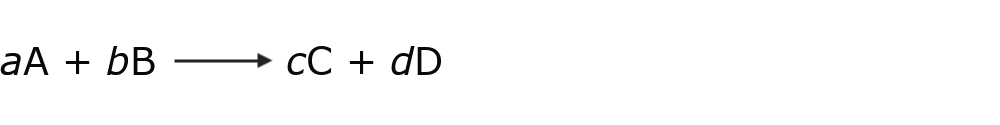
Where:
- A and B are the reactants.
- C and D are the products.
- to, b, c and d They are the stoichiometric coefficients (they are numbers that indicate the amount of reactants and products) that must be adjusted so that there is the same amount of each element in the reactants and products. In this way, the Law of Conservation of Mass is fulfilled (which establishes that mass is neither created nor destroyed, it is only transformed).
Types and examples of chemical reactions
Chemical reactions can be classified according to the type of reactants that react. Based on this, Inorganic chemical reactions can be distinguished from organic chemical reactions. But first, it is important to know some of the symbols used to represent these reactions through chemical equations: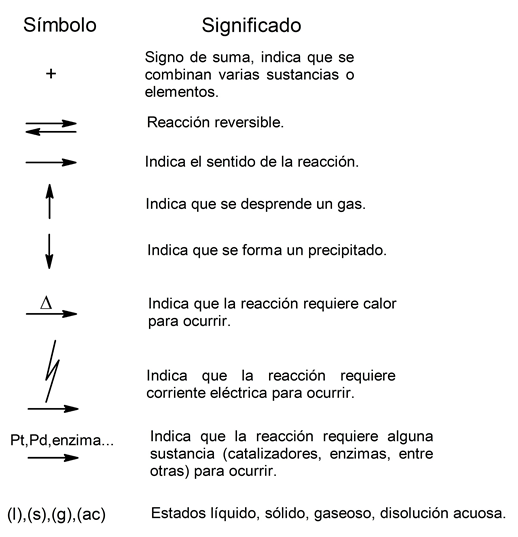

Inorganic reactions
They involve inorganic compounds, and can be classified as follows:
Depending on the type of transformation
- Synthesis or addition reactions Two substances combine to produce a different substance. For example:
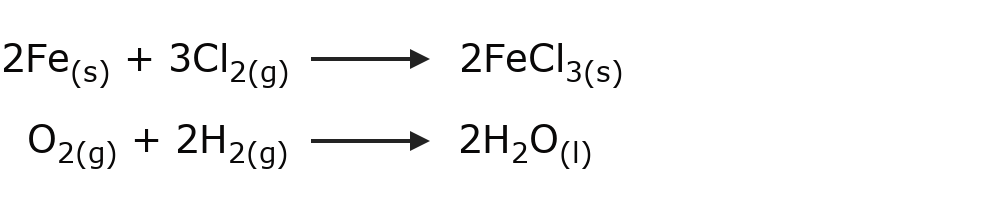

- Decomposition reactions A substance decomposes into its simple components, or one substance reacts with another and decomposes into other substances that contain its components. For example:
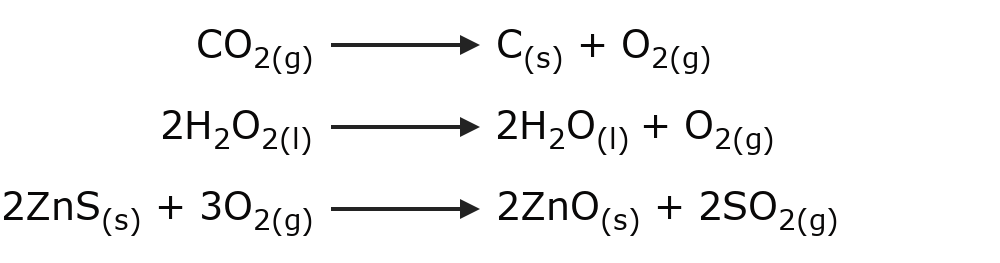

- Displacement or substitution reactions A compound or element takes the place of another in a compound, substituting it and leaving it free. For example:


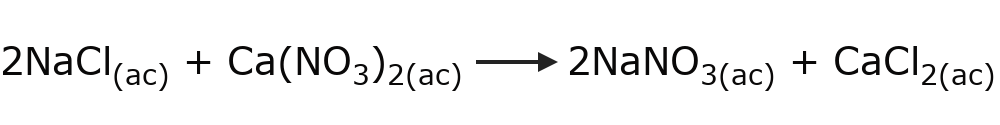
- Double substitution reactions Two reactants exchange compounds or chemical elements simultaneously. For example:

Depending on the type and form of the energy exchanged
- Endothermic reactions Heat is absorbed so the reaction can occur. For example:
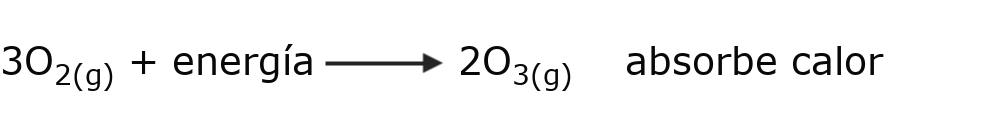
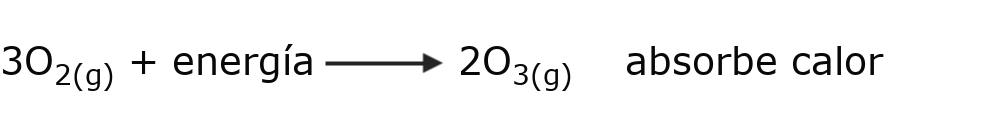
- Exothermic reactions Heat is released when the reaction occurs. For example:
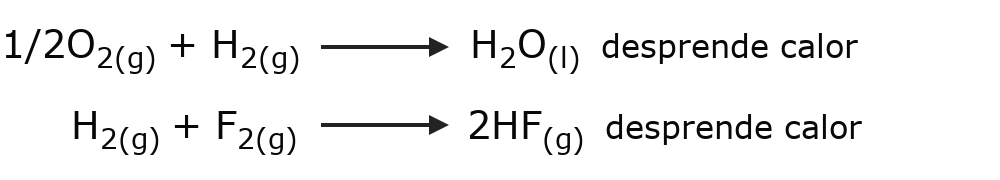

- Endoluminous reactions Light is needed for the reaction to occur. For example: photosynthesis.


- Exoluminous reactions Light is given off when the reaction occurs. For example:
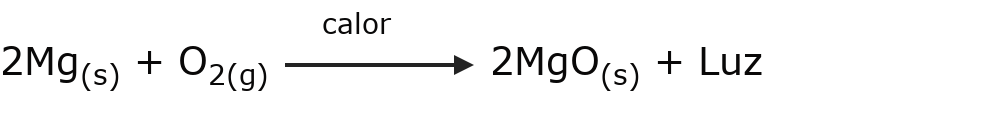
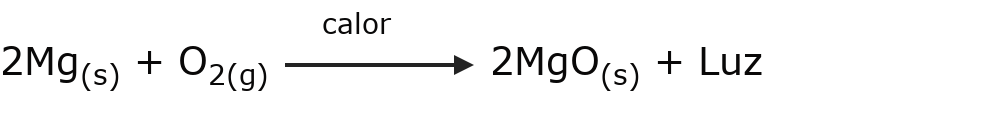
- Endoelectric reactions Electrical energy is needed for the reaction to occur. For example:
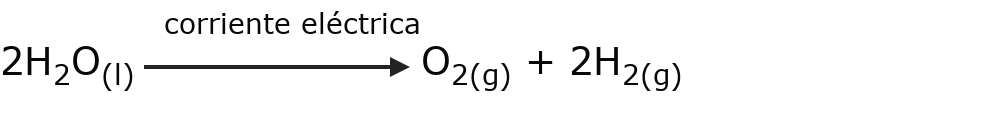
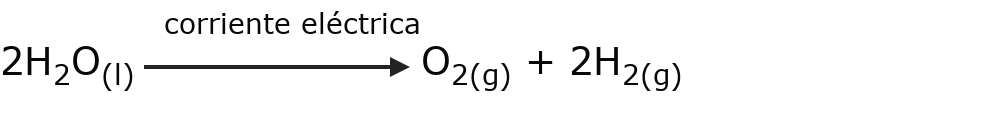
- Exoelectric reactions Electrical energy is released or generated when the reaction occurs. For example:
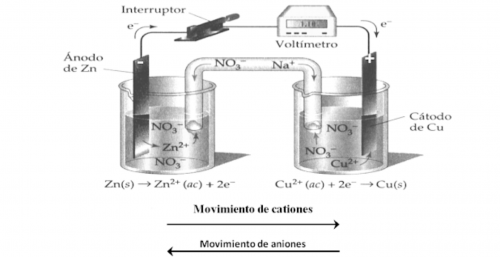

Depending on the reaction speed
- Slow reactions The amount of reactants consumed and the amount of products formed in a given time is very small. For example: the oxidation of iron. It is a slow reaction, which we see every day in iron objects that are rusty. If this reaction were not slow, we would not have very old iron structures in the world today.


- Quick reactions The amount of reactants consumed and the amount of products formed in a given time is large. For example: the reaction of sodium with water is a reaction that, in addition to occurring quickly, is very dangerous.


Depending on the type of particle involved
- Acid-base reactions Protons are transferred (H+). For example:
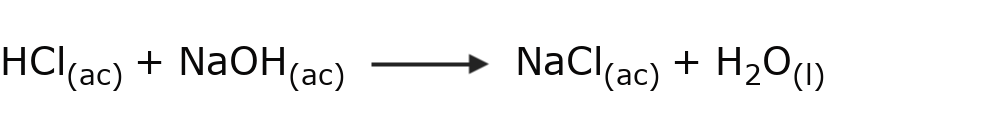

- Oxidation-reduction reactions Electrons are transferred. In this type of reaction we must look at the oxidation number of the elements involved. If the oxidation number of an element increases, it is oxidized, if it decreases, it is reduced. For example: in this reaction iron is oxidized and cobalt is reduced.


According to the direction of the reaction
- Reversible reactions They occur in both directions, that is, the products can become the reactants again. For example:


- Irreversible reactions They occur in only one direction, that is, the reactants are transformed into products and the opposite process cannot occur. For example:


Organic reactions
They involve organic compounds, which are those related to the basis of life. They depend on the type of organic compound for their classification, since each functional group has a range of specific reactions. For example, alkanes, alkenes, alkynes, alcohols, ketones, aldehydes, ethers, esters, nitriles, etc.
Some examples of reactions of organic compounds are:
- Halogenation of alkanes One hydrogen of the alkane is replaced by the corresponding halogen.
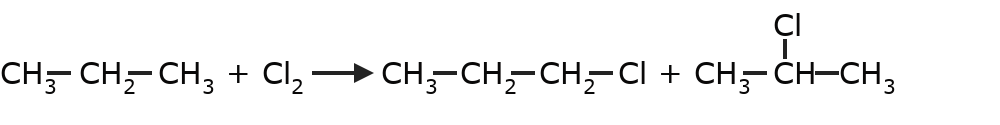
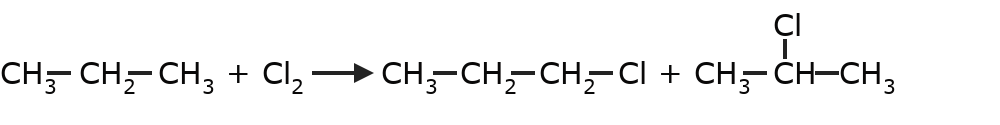
- Combustion of alkanes Alkanes react with oxygen to give carbon dioxide and water. This type of reaction releases a large amount of energy.
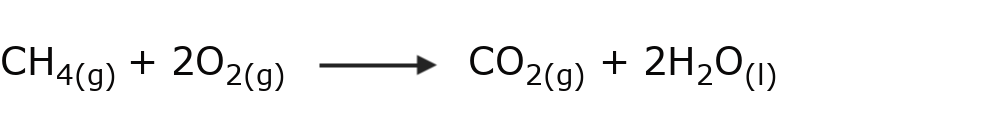
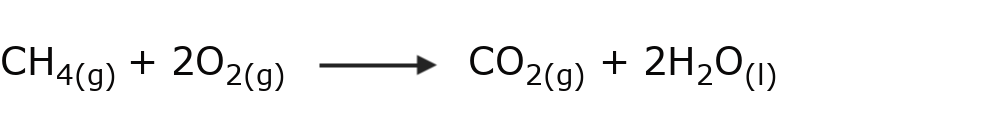
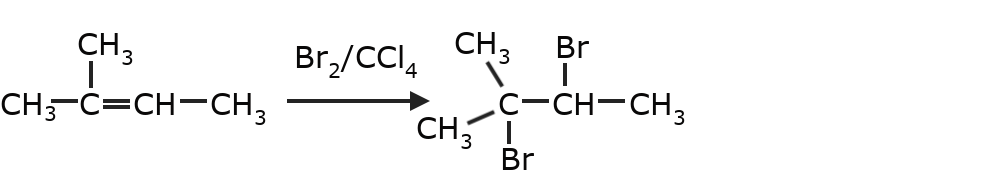
- Halogenation of alkenes Two of the hydrogens present on the carbons that form the double bond are replaced.

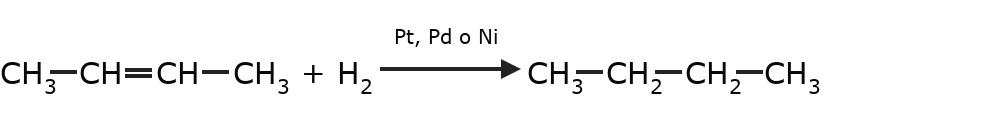
- Hydrogenation of alkenes Two hydrogens are added to the double bond, thus producing the corresponding alkane. This reaction occurs in the presence of catalysts such as platinum, palladium or nickel.

Importance of chemical reactions
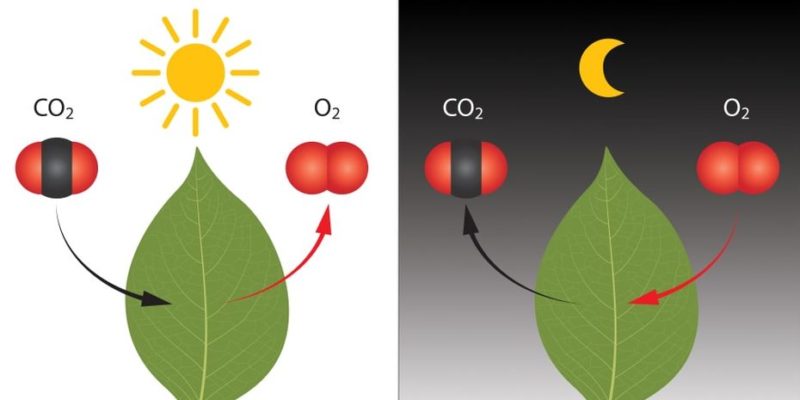
Chemical reactions are fundamental to the existence and understanding of the world as we know it. The changes that matter undergoes under natural or man-made conditions (and which often generate valuable materials) are just one example of this. The greatest evidence of the importance of chemical reactions is life itself, in all its expressions.
The existence of living beings of all types is only possible thanks to the reaction capacity of matter, which allowed the first cellular forms of life to exchange energy with their environment through metabolic routes, that is, through sequences of chemical reactions that released more useful energy than they consumed.
For example, in our daily lives, respiration is composed of multiple chemical reactions, which are also present in plant photosynthesis.
Speed of a chemical reaction
Chemical reactions require a stipulated time to happen, which varies depending on the nature of the reactants and the environment in which the reaction occurs.
The factors that affect the rate of chemical reactions generally tend to be:
- Temperature increase High temperatures tend to increase the speed of chemical reactions.
- Increase in pressure Increasing pressure usually increases the speed of chemical reactions. This generally occurs when substances that are sensitive to changes in pressure react, such as gases. In the case of liquids and solids, changes in pressure do not cause significant changes in the rate of their reactions.
- State of aggregation in which the reactants are found Solids usually react more slowly than liquids or gases, although the speed will also depend on the reactivity of each substance.
- Use of catalysts (substances used to increase the speed of chemical reactions) These substances do not intervene in the reactions, they only control the speed at which they occur. There are also substances called inhibitors, which are used in the same way but cause the opposite effect, that is, they slow down the reactions.
- Luminous energy (Light) Some chemical reactions are accelerated when light shines on them.
- Concentration of the reactants Most chemical reactions occur faster if they have a high concentration of their reactants.
Continue with: Redox reactions
References
- “Chemical reaction” https://es.wikipedia.org/
- “Types of chemical reactions” (video) Educatina https://www.youtube.com/
- “Chemical reactions” ICT Resources http://recursostic.educacion.es/
- “Chemical reactions” Khan Academy (English). https://www.khanacademy.org/
- “Chemical reactions” http://www.chem4kids.com/
- “Chemical reaction” https://www.britannica.com/
- “Inorganic Chemistry” Loyola, María Dolores de la Llata, Editorial Progreso. (2001) ISBN 9789706413512.
- “Chemistry” Eighth edition, page 304. Google Books.





