We explain what a compound is and some examples of this term. Also, the meaning of compound in various areas.
What is compound?
In chemistry, a compound is called a substance that is made up of two or more elements of the periodic table. The word compound comes from Latin composĭtus. We can talk about something being “composed of” to indicate what things make up something.
Chemical compounds have a chemical formula. A chemical compound is made up of molecules or ions that are stably linked. The chemical elements that make up a chemical compound cannot be separated with any physical treatment or process, but only with some chemical method.
A chemical compound should not be confused with a mixture (material formed by two or more components not chemically combined) or an alloy (mixture of two or more components where at least one is a metal). The components of a mixture or alloy can be separated using physical separation methods such as filtration, distillation, decantation and evaporation.
Chemical compounds are classified as organic and inorganic:
Inorganic compounds Inorganic chemistry is the branch of chemistry that deals with studying the properties and reactions of inorganic compounds, which can be classified into:
- Acid oxides They are non-metallic oxides. They are made up of a non-metal and oxygen. For example: Dichlorine trioxide (Cl2EITHER3) and sulfur dioxide (SO2).
- Basic oxides They are metal oxides. They are composed of a metal and oxygen. For example: calcium oxide (CaO) and iron (III) oxide (Fe2EITHER3).
- Hydrides They are made up of a chemical element and hydrogen. They can be metallic and non-metallic. For example: Calcium hydride (CaH2) and hydrogen fluoride (HF(g)).
- Hydracids They are non-metallic hydrides that, dissolved in water, become acids. For example: hydrofluoric acid (HF(ac)) and hydrochloric acid (HCl(ac)).
- Hydroxides They are produced by the reaction of a basic oxide and water. For example: sodium hydroxide (NaOH) and magnesium (II) hydroxide (Mg(OH)2).
- Oxacids They are produced by the reaction of an acid oxide and water. For example: Sulfuric acid (H2SW4) and perchloric acid (HClO4).
- Binary salts They are produced by the reaction between a hydracid and a hydroxide. For example: sodium chloride (NaCl) and iron (III) chloride (FeCl3).
- Oxysals They are produced by the reaction between an oxacid and a hydroxide. For example: iron (II) sulfate (FeSO4) and iron (III) sulfate (Fe2(SW4)3).
Organic compounds Organic chemistry is the field of chemistry responsible for studying the properties and reactions of organic compounds, which can be classified into:
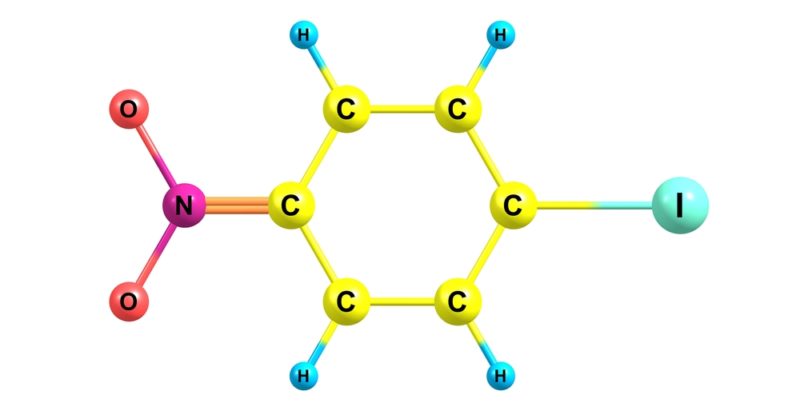
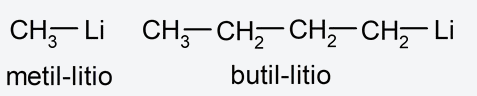
- Aliphatic compounds They are made up of carbon and hydrogen. They are not aromatic. For example:


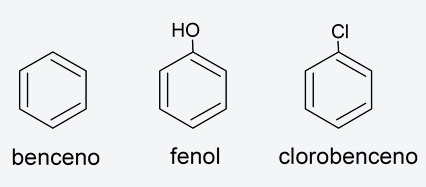
- aromatic compounds They are very stable cyclic organic compounds that have alternating single and multiple (double or triple) bonds in their structure. They are conjugated compounds, so named due to their structure. For example:

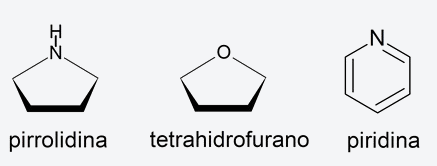
- Heterocyclic compounds They are cyclic organic compounds in which at least one atom of the cycle is different from carbon. For example:

- Organometallic compounds They are organic compounds in which carbon atoms form covalent bonds with metal atoms. For example:

- Polymers. They are macromolecules formed by the union of smaller molecules called monomers. For example: DNA.
Compounds in other areas
- Grammar. Compound words are those that are made up of simple terms. For example: remote-control.
- Botany. Composite plants are those that have simple leaves and flowers grouped in a common receptacle.
- Economy. Compound interest is the money, profit or utility of an initial capital at an interest rate during a certain period of time in which, when the interest generated is finished, it is reinvested.