We explain what decanting is and the ways in which it can be carried out. Also, some examples and separation methods.

What is decantation?
A physical procedure that It is used to separate a heterogeneous mixture composed of a solid or a liquid of higher density and a liquid of lower density.
This procedure consists of separating the densest component (solid or densest liquid) from the less dense liquid, by the action of gravity. The mixture is allowed to settle in a container (separating or settling funnels are generally used) and, in this way, the densest component descends by gravity towards the bottom of the funnel and the less dense component remains at the top.
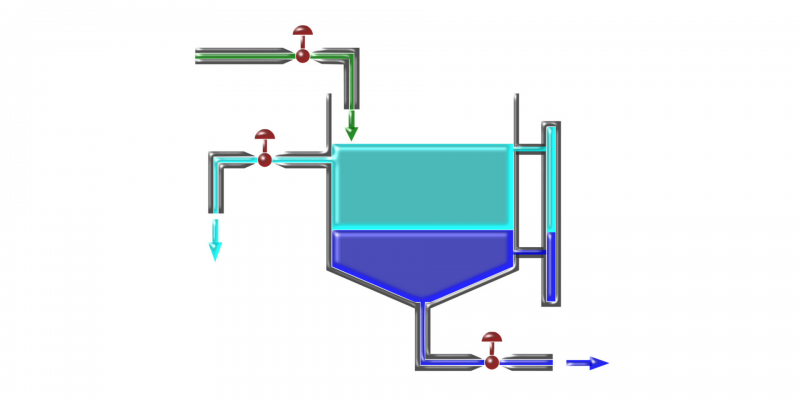
Finally, the densest component is extracted from the bottom. These types of mixture separation tools generally have several keys attached in different positions, which can be opened and closed to extract the desired component. A demonstrative example is shown in the following figure, where the mixture is green, the dark blue liquid is the most dense component and the light blue liquid is the least dense component.

This process It is widely used in laboratories and industrial processes especially using separatory funnels or similar tools. It should not be confused with sedimentation or gravitational separation, which consists of the separation of solid waste suspended in a liquid (such as water and sand) by the mere effect of gravity.
There are two ways in which decantation can be carried out, depending on the state of aggregation of the substances that make up the mixture:
- Solid-liquid decantation When solid elements are found deposited in a liquid medium.
- Liquid-liquid decantation When the mixture is formed by two liquids of different densities that cannot dissolve in each other.
Examples of decantation

- Wastewater treatment. Dirty water from the sewage system of cities is generally denser than clean water, since it is full of organic matter and other suspended substances. Therefore, an initial process of decanting and filtering the dirt can be carried out before returning the water to the seas and rivers. Thus, cleaner water is returned to the environment, reducing environmental pollution.
- Obtaining natural oils. It is known that fats are not soluble in water and, therefore, during the extraction of vegetable oils, decantation is usually used as a way to separate them from water. In addition, decanting is also used to extract solid residues remaining in the oil after crushing the palm, seed or olive.
- Separation of biodiesel and glycerin Glycerin is an unwanted byproduct of the process of obtaining biofuels that uses vegetable or animal fats. To separate them, the difference in densities is used in a decantation stage, which deposits the glycerin at the bottom of the container.
- Water purification In the processes to make water drinkable, various initial stages of decantation are used, which give the clays and other suspended materials time to settle to the bottom and be extracted, leaving the water cleaner for later filtering stages.
- Wine decanting. Wine is obtained from the fermentation of grapes, so it is necessary to separate the liquor from the physical residues that remain and that can often re-form in the bottle, after some time of sedimentation. Experts in the field then recommend applying a decanting process to extract the sediments and also oxygenate the wine. It is common in long-maturing wines.
- Manufacture of vinegars. The refinement of vinegar of vegetable origin requires decanting stages, in which it is separated from the heavier vegetable oils (also valuable), obtained during the transformation of the raw material.
- Refining oil . Oil must be refined to reach the necessary degree of hydrocarbon purity that allows obtaining its derivatives.. Various useful hydrocarbons result from this process, in gaseous or liquid state, which can be separated from the rest of the mixture by decantation. Thus, the lightest hydrocarbons are extracted and the densest ones continue to be refined at the bottom of the deposits.
- Oil extraction on maritime platforms. When extracting oil from deposits on the seabed, it usually happens that the hydrocarbon is mixed with seawater. This is remedied by decanting the mixture, since oil is much denser than water. The latter is stored and the water is returned to the ocean instead
Mixture separation methods
Mixture separation methods, also called phase separation methods, are the different physical (never chemical) procedures that allow you to separate the different components of a mixture using the different physical properties that each one presents.
So, for these methods to work, we must be in the presence of mixtures whose components retain their identity in the mixture and no chemical reactions take place that permanently alter their properties as a result of mixing them.