We explain what distillation is, examples of this separation method and the types of distillation that can be used.

What is distillation?
Distillation is called a phase separation method which is among the methods of separation of mixtures. Distillation consists of the consecutive and controlled use of two physical processes: vaporization and condensation, using them selectively to separate the components of a mixture that is generally homogeneous, that is, in which they cannot be distinguished with the naked eye. its components.
Mixtures that can be separated into their individual components using distillation may contain two liquids, a solid in a liquid, or even liquefied gases. This separation method It is based on the difference in boiling points (inherent property of matter, which is the temperature at which the vapor pressure of a liquid becomes equal to the pressure surrounding the liquid) of different substances. The substance with the lowest boiling point will first pass into the vapor phase, then this substance will condense in another container, and will remain relatively pure.
In this way, for the distillation to be carried out correctly, we must boil the mixture until the boiling point of one of the constituent substances is reached, which will then become vapor and can be taken to a cooled container, in which it will be It condenses and becomes liquid again.
The other component substance, on the other hand, will remain in the container without alterations; but in both cases we will have pure substances, free of the initial mixture.
Raoult's Law
When we have an ideal mixture of liquids (a mixture in which the interactions between different particles are considered equal to the interactions between equal particles) Raoult's Law is fulfilled.
This law states that the partial vapor pressure of each component in the gas mixture is equal to the vapor pressure of the pure component multiplied by its mole fraction in the liquid mixture.
The total vapor pressure is then the sum of the partial pressures of the components of the mixture in the gas phase. On the other hand, the mole fraction of a component in a mixture is a dimensionless measure of its concentration. The quantities mentioned above can be calculated using the following equations: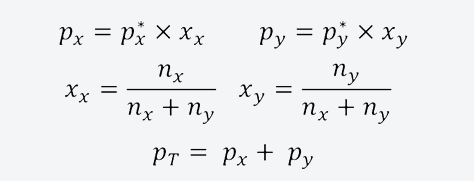
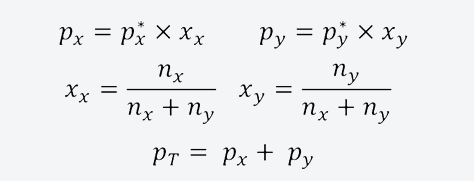
Where:
- px and pand are the partial pressures of the components x and and respectively in the vapor mixture that surrounds the liquid mixture.
- px*ypand* are the vapor pressures of the components x and and.
- xx and xand are the mole fractions of the components x and and in the liquid mixture.
- nx and nand are the amount of substance of the components x and and in the liquid mixture.
Raoult's Law stated above is valid for ideal mixtures (which are a model established by humans to simplify studies), but in reality this law suffers deviations when the mixture is real.
Thus, if the different particles in the mixture have stronger intermolecular forces than the particles in the pure liquid, then the vapor pressure of the mixture is less than the vapor pressure of the pure liquid, which produces a negative deviation from the Law by Raoult.
Yes, on the other hand, The intermolecular forces between the particles in the pure liquid are greater than those between the particles in the mixture the particles in the mixture will be able to escape to the vapor phase more easily, so the vapor pressure of the mixture will be higher, producing a positive deviation from Raoult's law
When you want to distill an azeotropic mixture (for example, ethanol and water), it is necessary to add some component (benzene, in this case) so that the azeotrope is modified and in this way the components of the mixture can be separated. An azeotrope is a liquid mixture with a defined composition that when it boils, the vapors generated have the same composition of the mixture (so the components of the azeotropic mixture cannot be separated by simple or fractional distillation).
Azeotropes have a defined boiling temperature, for example, at a pressure of 1 atm, ethanol boils at 78.37 °C and water boils at 100 °C, while the ethanol-water azeotrope boils at 78.2 ° c. Azeotropic mixtures have negative or positive deviations from Raoult's Law as the case may be.
Types of distillation
Distillation can occur in different ways:
- Simple distillation. The most basic is to boil the mixture until the different components are separated. It is an effective separation method when the boiling points of the components of the mixture differ greatly (ideally, they should have a difference of at least 25°C, otherwise it does not guarantee the total purity of the distilled substance).
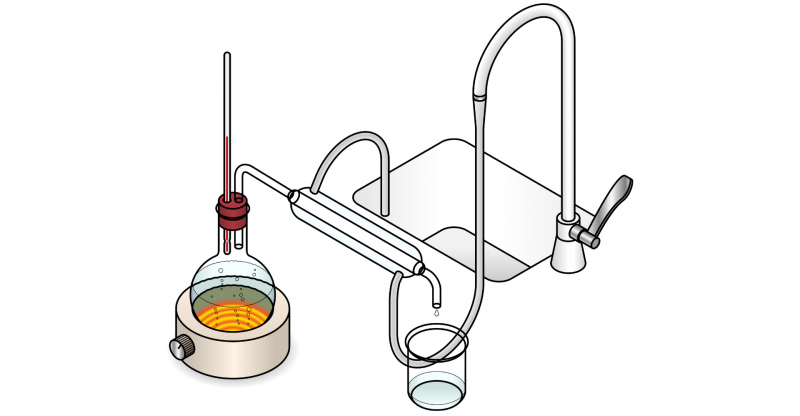

- Fractional distillation It is carried out using a fractionation column, which is made up of different plates in which vaporization and condensation occur successively, guaranteeing greater purity in the separated components.

- Vacuum distillation By reducing the pressure until a vacuum is generated, the process is catalyzed to reduce the boiling point of the components, since some have very high boiling points that can be reduced when the pressure is greatly reduced and, in this way, accelerate the process of distillation.
- Azeotropic distillation It is the distillation necessary to break an azeotrope, that is, a mixture whose substances behave as one, even sharing the boiling point, so they cannot be separated by simple or fractional distillation. To separate an azeotropic mixture, it is necessary to modify the conditions of the mixture, for example, by adding a separator component.
- Steam distillation. The volatile and non-volatile components of a mixture are separated by direct injection of water vapor.
- Dry distillation. It consists of heating solid materials without the presence of liquid solvents, obtaining gases and then condensing them in another container.
- Improved distillation. Also called alternate or reactive distillation, it is adapted to the specific cases of mixtures that are difficult to separate or have the same boiling point.
Examples of distillation

- Oil refining. The separation of the various hydrocarbons present in oil is carried out through fractional distillation, storing each compound derived from the cooking of crude oil in various layers or separate compartments of a distillation column. These gases rise and condense in the upper layers of the column, while denser substances such as asphalt and paraffin remain in the lower layers.
- Catalytic cracking This is the name given to certain vacuum distillations common in oil processing, using vacuum towers to separate the cooking gases from the crude oil. This accelerates the boiling of the hydrocarbons and speeds up the process. Cracking is a type of destructive distillation, in which larger hydrocarbons are broken down (at high temperatures and using catalysts) into smaller hydrocarbons, which have a lower boiling point.
- Purification of ethanol. To separate alcohols such as ethanol from water during their production in the laboratory, azeotropic distillation is used, adding benzene or other components to the mixture that promote or accelerate the separation, and which can then be easily removed without altering the chemical composition of the product.
- Coal processing. To obtain liquid organic fuels, coal or wood is used through dry distillation procedures, thus the gases emitted in combustion can be condensed.
- Thermolysis of mineral salts. Through dry distillation, various mineral substances of high industrial utility are obtained from the emanation and condensation of gases obtained by burning mineral salts.
- The alembic. This is the name given to the device invented in Arab antiquity, whose purpose is to produce perfumes, medicines and alcohol from fermented fruits. In its operation, the principles of distillation are used: substances are heated in a small boiler and the gases produced are cooled in a coil that leads to another container where the liquid produced by the condensation of said gases is collected.
- Perfume production. Steam distillation is used to obtain perfumes, boiling water together with preserved flowers, to produce a gas with the desired smell and which then, when condensed, can be used as a base liquid in perfumes.