We explain what filtration is and the types that exist. Also, some examples and methods of separating mixtures.

What is filtration?
It is known as filtration a technique for separating suspended solids within a fluid (liquid or gas) using a filter medium that consists of a porous material called a sieve, filter or sieve. This filter retains larger solids and allows the fluid to pass through, along with smaller particles.
The filtration process is, therefore, very similar to that of sieving, except that the latter is used to separate solid materials of different thickness or size. The filtration It is part of the most used mixture separation methods in the daily life of human beings. It is also a method widely used in different types of industries, so there are different mechanical devices capable of carrying it out with a varied range of precision.
Commonly, we talk about leaks in everyday life when we refer to excess humidity that softens cement and other construction materials, allowing water to flood the pores of walls, ceilings and floors, making its way through and deteriorating them. In that case, it is the water that leaks through the cement, and colloquially it is often said “there are leaks in the walls or ceiling.”
See also: States of matter
Filtration types
In the filtration process What remains in the filter is called “residue”, and what passes through it is called “filtered”. The filter material that makes up the filter is essential to ensure that the filtration works properly for the specific objectives. There are various types of porous materials used as filter media, for example, fabrics, porous or perforated solids, polymeric fibers. On the other hand, depending on the size of the pores of the filter material used, the filtration process can be classified into different types:
- Ordinary filtering It is the process that is carried out with membranes or sieves whose pores are equal to or greater than one millimeter (mm).
- Microfiltration It is the type of filtration that is carried out with sieves whose pores range between 0.1 and 10 microns (1 mm = 1000 microns).
- Ultrafiltration It is the filtration process that retains molecules whose weight exceeds 103 Dalton/gmol, allowing you to separate proteins or disinfect water with bacteria. Thus, this type of filtration allows particles with a diameter of up to 0.01 micron to be filtered.
- Nanofiltration This process allows molecules without an electrical charge that have a weight greater than 200 Dalton/gmol to be retained in the filter membrane, and is applied in the chemical industry mainly to concentrate organic compounds.
Filtration Examples

Some everyday examples of filtration can be:
- Prepare coffee. To make coffee or other infusions, the substance (tea, coffee, etc.) is brought into contact with boiling or very hot water, to force it to release its contents into the water. Then they must be separated, and to do so, the mixture is poured into a cloth or paper filter, which retains the coarsest particles of the infusion (the so-called “coffee grounds”) and the liquid is allowed to pass through.
- Leaks due to broken pipes Water from a broken pipe can accumulate and seep through the cement with which buildings are made, softening its consistency and making its way downwards due to gravity, or upwards due to pressure. In both cases, the water seeps through the cement, leaving any particles on the other side of the wall.
- Water purifiers Water filters have operated for centuries by sieving drinking water, either using especially porous stones (as in tinajeros or jars) or using papers, corks and other solids that serve to retain the particles that the water brings with it. In this way, the water is as clean as possible.
- Strain the pasta. When we make pasta or spaghetti, we boil the food in water and then separate it using a strainer, which is nothing more than a coarse filter. Thus, it is possible to retain the cooked pasta and discard the hot water.
water filtration

Water filtration is a necessary process to guarantee its minimum drinkability That is, it does not contain stones, earth, metals or other waste materials that it may have dragged on its way to our homes.
For this Filtering devices or mechanisms installed in the pipe itself are used which retain dirt and solid materials, allowing water to pass through their porous bodies. This mechanism does not prevent against microorganisms, so it only guarantees a first sanitary measure for water consumption.
Mixture separation methods

Filtering is just one of the methods for separating mixtures, that is, one of the procedures we have when separating two or more mixed substances. Other methods of separation of mixtures are:
- Decantation It is a physical separation method that consists of waiting for gravity to act on the solids present in a fluid, allowing them to settle and be removed mechanically. It can also be used to separate a mixture of two liquids that have different densities, that is, they are immiscible with each other. In this case, the mixture contained in a separator system (for example, a separating funnel) is allowed to rest, where the denser liquid passes to the bottom, and the less dense liquid remains at the top, making the separation of both possible.
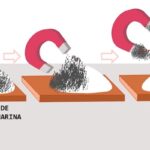
- Magnetic separation. To separate metals or magnetic particles from a liquid or another solid, a magnet can be used since it will attract only the metallic particles, and will leave the diamagnetic particles intact (particles that repel magnetic fields, that is, they are not attracted by the magnetic field. which generates a magnet).
- Distillation. It is a procedure that consists of separating the components of a liquid mixture using the difference between their boiling and condensation points. Thus, heat is supplied to the mixture until the component with the lowest boiling point passes into the vapor phase. Then this vapor condenses in another container, and the liquid with the highest boiling point remains in the original container. In this way, both liquids are separated.
- Evaporation It is a process similar to distillation. Allows you to separate mixtures of solids into liquids. In this case, the liquid in which the solid is dissolved is evaporated, and thus the solid can be recovered at the bottom of the container. This process is used in salt flats to separate the salt that we later use in our meals (NaCl) from seawater.
References
- «Chemical Engineering: Basic Operations» SI Units. Volume II. John Metcalfe Coulson, John Francis Richardson, JR Backhurst and JH Harker. Reverte Publishing (2003). ISBN: 8429171363, 9788429171365.