We explain what an ionic bond is and its properties. Examples and applications of compounds formed with this type of bonds.
What is an ionic bond?
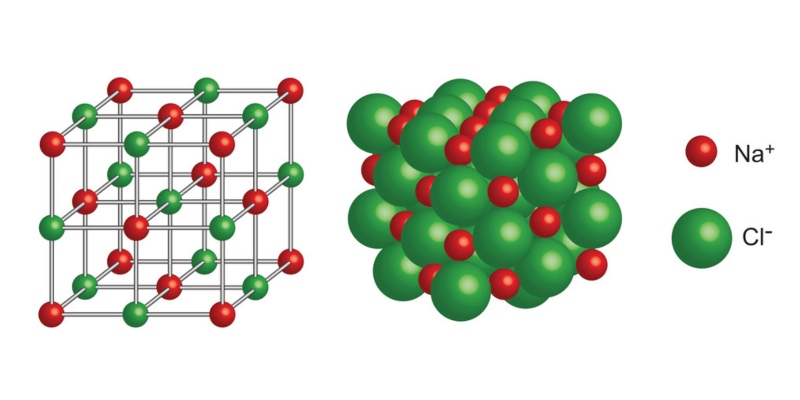
The ionic or electrovalent bond consists of the electrostatic attraction between particles with opposite electrical charges called ions.
An ion is an electrically charged particle. It can be an atom or molecule that lost or gained electrons, that is, it is not neutral.
This type of link It generally occurs between metallic and non-metallic atoms in which the transfer of electrons occurs from metallic atoms (less electronegative) to non-metallic atoms (more electronegative).
For an ionic bond to form, it is necessary that the difference in electronegativity (ability of an atom to attract electrons from another atom when combined in a chemical bond) between both types of atoms is greater than or equal to 1.7 on the scale of Pauling, used to classify atoms according to their electronegativity values.
Although the ionic bond is usually distinguished from the covalent bond (consisting of sharing electronic pairs of the outer or valence shell of both atoms), in reality there is no pure ionic bond Rather, this model consists of an exaggeration of the covalent bond, useful for the study of atomic behavior in these cases. There is always some margin of covalence in these unions.
However, unlike the atoms that form the covalent bonds that often constitute polar molecules, ions do not have a positive and a negative pole, but rather in them a single charge predominates entirely. Thus, we will have cations when an atom loses electrons (remains positively charged) and anions when an atom gains electrons (remains negatively charged).
Properties of ionic compounds
Some general characteristics of an ionic compound:
- are strong links The strength of this atomic bond can be very intense, so the structure of these compounds tends to form very resistant crystalline networks.
- are usually solid At normal temperatures and pressure ranges (T=25ºC and P=1atm), these compounds have a cubic and rigid molecular structure, which forms crystalline networks that give rise to salts. There are also ionic liquids called “melt salts,” which are rare, but extremely useful.
- have a high melting and boiling point Both the melting point (between 300 ºC and 1000 ºC) and the boiling point of these compounds are usually very high, since large amounts of energy are required to break the electrostatic attraction between the ions.
- Solubility in water Most salts are soluble in water and other aqueous solutions that have an electric dipole (positive and negative poles).
- Electric driving In their solid state they are not good conductors of electricity, since the ions occupy very fixed positions in a crystalline lattice. On the other hand, once dissolved in water or an aqueous solution, they become effective conductors of electricity.
- Selectivity Ionic bonds can only occur between metals from groups IA and IIA of the Periodic Table, and non-metals from groups VIA and VIIA.
Examples of ionic bond
- Fluorides (F–) Anions that are part of salts obtained from hydrofluoric acid (HF). They are used in the manufacture of toothpastes and other dental supplies.
Examples: NaF, KF, LiF, CaF2
- Sulfates (SO42-). Anions that are part of salts or esters obtained from sulfuric acid (H2SW4), whose union to a metal has diverse applications, from additives in obtaining construction materials, to supplies for contrast x-rays.
Examples : CuSO4Case4K.2SW4
- Nitrates (NO3–) Anions that are part of salts or esters obtained from nitric acid (HNO3), used in the manufacture of gunpowder and in numerous chemical formulations for fertilizers.
Examples: AgNO3K.N.O.3Mg(NO3)2
- Mercury II (Hg2+) A cation obtained from mercury, also called mercuric cationand that it is only stable in acidic pH media (<2). Mercury compounds are toxic to the human body, so they must be handled with certain precautions.
Examples: HgCl2HgCN2
- Permanganates (MnO4–) Salts of permanganic acid (HMnO4) have an intense purple color and enormous oxidizing power. These properties can be used in the synthesis of saccharin, in the treatment of wastewater and in the manufacture of disinfectants.
Examples: KMnO4Ca(MnO4)2