We explain what magnetic separation is, its characteristics, examples and other mixture separation techniques.
What is magnetic separation?
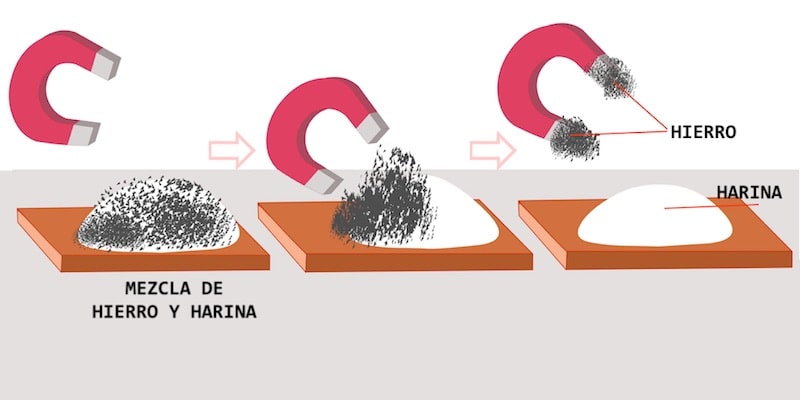
Magnetic separation is a physical method of separating mixtures, which uses magnetic susceptibility of some of its components. To do this, expose the mixture to a magnet (or a sufficiently intense magnetic field) for a period of time.
In other words, the technique It consists of bringing a magnet close to the mixture, to attract the ferromagnetic components of the mixture, leaving the non-magnetic ones in the container.
magnetism manifests itself in a force of attraction or repulsion depending on the polarity of the opposing magnetic fields: like poles repel each other, different poles attract each other. It is a property of matter that is present in all matter that is more noticeable in some materials than in others.
The magnetic separation technique is widely used in recycling or rescue of certain metallic elements, which differ from their environment in terms of their molecular nature. There are even variants that can be applied to polar organic substances, but these are more delicate procedures.
Magnetic Separation Features

magnetic separation It works based on the enormous difference in magnetic susceptibility that there may be between the components of a mixture. All those that respond to magnetism will undergo modification when exposed to a magnet or electromagnet, leaving the rest in place.
Obviously, the ideal would be that not all the components of the mixture are magnetic or not all to the same extent, so that through controlled exposure to magnetic forces, the mixture can be effectively separated.
Examples of magnetic separation
This technique is very useful to separate mixtures such as:
- iron filings present in flour, sulfur, gravel or other solid materials.
- Coins clips, metal objects from the beach sand.
- solid nickel among other less magnetic metals, such as bronze or silver.
Other mixture separation methods

As well as magnetic separation, there are other physical and chemical methods to separate mixtures, such as:
- Filtered. Useful for separating insoluble solids from liquids, it consists of the use of a filter (filter paper, filter stones, etc.) that allows the liquid to pass but retains the solid elements.
- Decantation Used to separate liquids that do not dissolve in each other, or insoluble solids in a liquid, using an ampoule or a separatory funnel, where the mixture is allowed to rest until the densest component goes to the bottom, while the less dense remains on the surface.
- Sifted. It is a separation method similar to filtering, but it is used to separate mixtures of solid substances of different sizes. It is made with a net or sieve, whose holes allow the smaller fragments to pass through and retain the larger ones.
- Distillation. It allows you to separate soluble liquids from each other that have different boiling points. The procedure consists of pouring the mixture into a container and heating it, controlling the temperature so that only the component with the lowest boiling point vaporizes and can be redirected to another container, where it condenses.
- Evaporation. It is used to separate solids dissolved in liquids and consists of evaporating the liquid until crystals of the dissolved solid are obtained at the bottom of the container.
Continue with: Chemical solution
References
- “Magnetic separation” on Wikipedia.
- “Magnetic separation technique” at Steinert Solutions.
- “Magnetic separation” in ICT Resources.
- “Magnetic Separation” (video) on TutorVista.
- “Magnetic Separation” in The Encyclopaedia Britannica.





