We explain what a mixture is, the characteristics of each type and various examples. Also, what are pure substances.

What is a mixture?
In chemistry, a mixture It is a material composed of two or more components joined together physically, but not chemically. This means that no chemical reaction occurs between them, that is, each component maintains its identity and chemical properties, even in the case where we cannot distinguish one component from the other.
The components or phases of a mixture are mechanically or physically linked. That's why, their physical properties are often altered such as boiling or melting point.
However, since permanent chemical changes do not occur, it is possible to use physical separation mechanisms to extract each of the components from a mixture. These physical mechanisms are usually thermal (when they involve heat) or mechanical (when they involve displacement or movement).
Mixtures are mixed forms of matter that are extremely common in everyday life, and many of the materials we use are the result of a mixing or mixing procedure. The components of a mixture can be found in different states of aggregation (solids, liquids, gases, plasmas, or combinations between them).
Types of mixtures
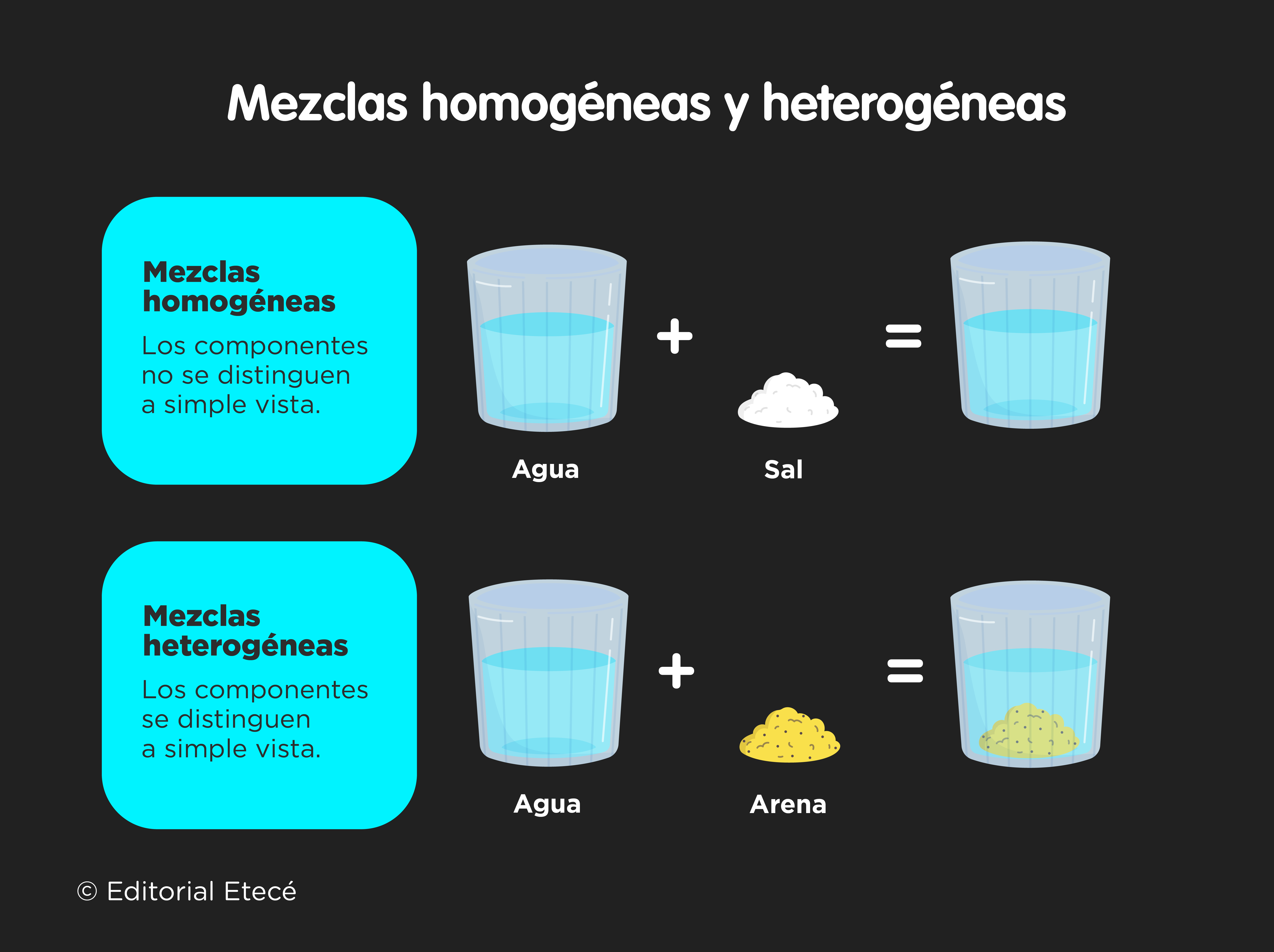
Mixtures are classified based on how feasible it is to identify their different components at a glance.
- Homogeneous mixtures They are those in which the components cannot be distinguished. They are also known as solutions, and are made up of a solvent and one or more solutes. And as we have said, the phases are impossible to identify with the naked eye.
- Heterogeneous mixtures They are those in which the components can be easily distinguished, because they have a non-uniform composition, that is, their phases are integrated unevenly and irregularly, and therefore it is possible to distinguish their phases with relative ease. Depending on the particle size of its components, we can talk about two types of heterogeneous mixtures:
- Coarse mixtures or coarse dispersions. They are those in which the size of the particles is noticeable with the naked eye.
- Suspensions or colloids. They are those in which one phase is normally fluid (gaseous or liquid) and the other is composed of particles (generally solid) that remain suspended and deposit over time.
Examples of homogeneous mixtures

Below we present some examples of homogeneous mixtures:
- The air The common gas that we breathe daily is a mixture of pure gaseous substances (such as oxygen, nitrogen and argon, among others) that are impossible to perceive with the naked eye and that, together, are usually odorless.
- Alcoholic beverages. Like cocktails, they consist of two or more liquids (or even solids) mixed until they acquire a uniform appearance and, although we can taste their components, we cannot identify them with the naked eye.
- Sugar water. What we usually give to people who decompensate is a dilution of a solid (sugar) in a liquid (water), to the point that the difference cannot be seen.
- Metal alloys. Like the stainless steel from which some knives are made, they are obtained by mixing iron with carbon and other metal components, so that the mixture acquires the combination of their properties. To do this, they must be melted to a liquid state, then mixed and allowed to solidify.
- The amalgams. As used by the dentist in ancient times, they were usually a mixture of mercury and some other metal, converted into a uniform and malleable paste, which then hardened as it solidified.
- The shaving foam. It is a mixture of water, soaps, glycerin and menthol, often accompanied by gases (if it comes in a spray).
- The blood It is also a homogeneous mixture of an immense number of liquid, solid and gaseous compounds, which we appreciate simply as a more or less thick red liquid.
Examples of heterogeneous mixtures

These are some examples of heterogeneous mixtures:
- Aerosols. Like deodorants or spray paint, they are made up of a mixture of liquid and gas, which are ejected from the container at the same time, but then the gas disperses and the liquid remains on the sprayed surface. It is a colloidal mixture.
- Gravel or gravel. It is a mixture of two or more types of stone in small pieces, which can be distinguished with the naked eye. It is a case of coarse dispersion.
- A salad It is another perfect example of coarse dispersion, since we can see each of its components with the naked eye but they all work together: vegetables, oil, fruits, sometimes meats, etc.
- Water and oil. It is also an example of a heterogeneous mixture in which we can identify both phases, although in this case it is a liquid-liquid suspension.
- Some medications In whose packaging it is suggested that we shake them before use, they are cases of suspensions in which the solid precipitates to the bottom over time, and that is why we must shake it so that it dissolves again, making the distinction between one and the other temporarily negligible.
- The concrete It is a mixture of water, sand and cement in specific proportions that, once solidified and dried, acquires its hardness and uniformity.
Continue in: Heterogeneous mixture
pure substances
The pure substances are those that are not the result of a mixture but are composed of a single phase and, therefore, cannot be separated into their components by physical methods. Furthermore, they have a stable chemical composition and are chemically uniform.
A pure substance does not necessarily have to be composed of a single type of chemical element. Pure substances can be classified into:
- simple substances. They are those composed of a single type of chemical element (which does not mean that they are composed of a single atom). For example: oxygen (O2), nickel (Ni).
- Compound substances. They are those composed of more than one type of chemical element. For example: water (H2O), carbon dioxide (CO2).
The only way to separate the elements of a pure substance is using chemical methods, that is, transforming it into other substances or directly into its chemical elements.
It is necessary to clarify that absolute purity does not exist. In the world we live in, substances exist in nature in the form of certain mixtures, or in other words, with a certain level of impurities. However, impurities can be separated until the desired or permitted degree of purity of the substance is achieved.
Examples of pure substances: copper (Cu), silver (Ag), gold (Au), glucose (C6h12EITHER6), oxygen (O2), water (H2EITHER).
References
- “Mixture” on Wikipedia.
- “Mixtures” in Cide@d of the Spanish Ministry of Education.
- “We evaluate: pure substances and mixtures” (video) in Science Bits.
- “Substances and mixtures” in TP Chemical Laboratory.
- “Materials and mixtures” in Educational Portal.





