We explain what a molecule is and examples of this set of atoms. Also, the types that exist and their difference from the atom.

What is a molecule?
A molecule is a set of atoms (of the same chemical element or of many different ones) that are organized and interrelated through chemical bonds. A molecule is also considered to be the smallest part of a substance that still retains the physical and chemical properties of the substance. The molecules are usually chemically stable and electrically neutral.
The state of aggregation of a substance depends mostly on the structure and types of atoms that make up its molecules, since these determine the strengths of the interactions between these particles. In this sense, solids are compounds that have very little separation between their molecules, liquids have a medium or intermediate separation between their molecules and gases have a lot of separation between their molecules.
The study of molecules and their nomenclature not only includes the number of atoms that compose them and the properties they present, but also their understanding based on a three-dimensional model of their bonds and structures, that is, the organization in the space of its constituent atoms. This means that there are molecules that have the same atomic composition but different spatial structures (and that is why these molecules are named differently).
The molecules They are very common in organic chemistry since they are part of atmospheric gases and oceans. However, there is a large number of chemical compounds in the Earth's crust that are not molecular. For example, most metals and minerals in the Earth's crust are not molecules. On the other hand, the crystals that make up the salts are not molecules either, despite being made up of repetitive units.
Examples of molecules
Some examples of common molecules are:
- Oxygen: EITHER2
- Hydrochloric acid: HCl
- carbon monoxide:CO
- Sulfuric acid:H2SW4
- Ethanol:C2h5OH
- phosphoric acid:H3PO4
- Glucose:C6h12EITHER6
- Chloroform: CHCl3
- Saccharose:C12h22EITHER11
- paraaminobenzoic acid:C7h7NO2
- Acetone:C3h6EITHER
- Cellulose:(C6h10EITHER5)n
- Trinitrotoluene:C7h5N3EITHER6
- Silver nitrate: AgNO3
- Urea: CO(NH2)2
- Ammonia: NH3
Types of molecules

Molecules can be classified according to the complexity of their constitution:
- Discrete molecules. They present a defined number of atoms, whether they are of the same elements or different chemical elements. They can be classified, in turn, according to the number of different atoms that make up their structure: monatomic molecules (the same type of atom), diatomic molecules (two types of atoms), triatomic molecules (three types of atoms), tetraatomic molecules (four types of atoms), etc.
- Macromolecules or polymers. This is the name given to large molecular chains. They are composed of simpler pieces, which are joined together to achieve extensive sequences and acquire new and specific properties. Plastics, for example, are a material composed of organic macromolecules.
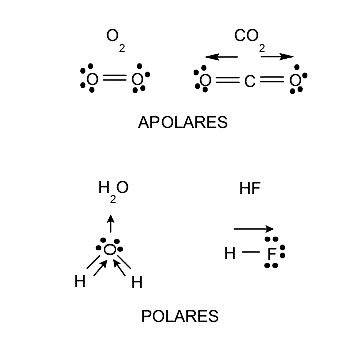
Polarity is a property that molecules have and is closely related to the separation of electrical charges that has or is generated within each molecule. This property influences solubility, since polar substances dissolve polar substances and nonpolar substances dissolve only nonpolar substances, although there are always intermediate situations. Melting and boiling points, and even aggregation states, are also affected by polarity. Therefore, molecules can also be classified according to their polarity into:
- polar molecules They are those formed by atoms with different electronegativity, that is, the atom with greater electronegativity attracts the electrons of the bond towards itself and with greater force, so there is a negative charge density around it. On the contrary, a positive charge density will remain on the less electronegative atom. This process will eventually lead to the formation of a dipole, which is a system of two charges of opposite sign and equal magnitude.
- Nonpolar molecules They are those whose atoms have identical electronegativity, that is, they do not present inequality with respect to the attraction of electrons and retain a neutral charge in an ordinary situation.
The symmetry of a molecule (the position that each of its atoms occupies in its structure) can also play a role in determining whether a molecule is polar or nonpolar. There are molecules composed of atoms of different electronegativity, but which are also nonpolar, because when the charge densities of various parts of the molecule are added, these charges are canceled, and the molecule is finally left with a neutral charge, that is, without electric charge.

Differences between atom and molecule
Molecules are made up of atoms joined together by chemical bonds, therefore, atoms are smaller particles than molecules. In fact, most molecules can undergo rupture procedures either lysis of their chemical bonds, transforming into simpler molecules, or into pure chemical elements, that is, atoms.
water molecule

A water molecule contains only two elements: one oxygen atom and two hydrogen atoms (H2EITHER) covalently linked. This was discovered in 1782 thanks to the chemist Henry Cavendish, since since ancient times water was thought of as an element.
Water has a non-linear structure. Its two hydrogen atoms are bonded to the oxygen atom and form an angle of 104.5º with each other. This distribution of its atoms, added to the high electronegativity value of the oxygen atom, generates the formation of an electric dipole that determines the polarity of water. Therefore, water is a polar molecule.
Water is considered the universal solvent, since almost all substances can dissolve in it. Substances soluble in water are polar and called hydrophilic. Non-polar (apolar) substances, such as oil or gasoline, are called hydrophobic and do not dissolve in water.
The water molecule, extremely abundant on our planet, It is also part of numerous organic substances and the bodies of animals and plants.
References
- «Sciences applied to professional activity 4th ESO» by Vaquerizo, Dulce María Andrés, Editex (2016). ISBN 9788490788097.
- “Atomic structure and chemical bond” by Jaime Casabó Gispert, Reverté (1996). ISBN 978-84-291-7189-1