We explain what organic chemistry is, its origin and relationship with inorganic chemistry. Also, classification of organic compounds.

What is organic chemistry?
Organic chemistry (also called carbon chemistry) is the study of organic substances and compounds which means that they have as the combinatorial basis of their atomic structure the elements carbon, hydrogen and some others such as sulfur and oxygen. Furthermore, organic compounds constitute the different forms of living beings on our planet.
In this sense, organic chemistry as a field of study is interested in the structure, behavior, properties and uses of this type of chemical compounds. That's why, It is essential to understand how life works and the various energy and industrial processes that the human species has developed throughout its history.
For modern chemistry, the elements that form organic compounds are those that usually appear in living organisms and their derived compounds, such as carbon (C), hydrogen (H), sulfur (S), oxygen (O ), nitrogen (N) and all halogen elements.
Although the elements mentioned are the most common, organic substances can also be composed of other elements, both organic and inorganic.
See also: Biochemistry
Origin of organic chemistry
The origin of the name “organic chemistry” comes from certain scientific theories that were in vogue until the mid-19th century and who proposed that organic compounds were, necessarily, remains or residues of ancient living beings. Therefore, they maintained that all organic matter came from their bodies.
However, in 1828 the German chemist Friedrich Wöhler realized that inorganic substances such as ammonium cyanate (CH4N2O) they could become through certain chemical processes, into an organic substance such as urea, which is part of the urine of numerous animals, for example.
Wöhler obtained the first evidence that organic and inorganic matter could have a common origin, not necessarily related to life.
organic chemistry It began to be a fundamental branch of modern chemistry in the 20th century when new research methods emerged thanks to technology. In this way, it was possible to better understand the processes of organic compounds. Biology and medicine also played an important role in this.
Classification of organic compounds
Organic compounds are roughly classified as follows:
Depending on the way they are produced or synthesized:
- Natural compounds They are those synthesized both by living organisms and by natural processes. In either of the two variants, humans do not intervene to synthesize them. For example: proteins, lipids and nucleic acids can be synthesized by living organisms, while oil can be produced as a result of geological processes that take thousands of years.
- Synthetic compounds They are artificially synthesized by humans in chemical laboratories. For example: drugs, dyes, plastics, among other products.
Depending on the type of structure:
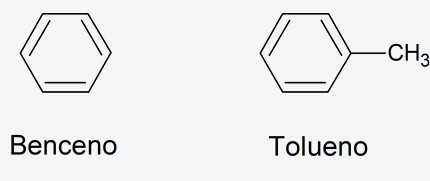
- Aromatic hydrocarbons They are cyclic organic compounds (ring-shaped) that have the peculiarity in their structure of alternating a single bond with a multiple bond, generally a double bond. The fact that the bonds alternate causes a delocalization of the electrons on the ring, which gives great stability to this type of structure. Most are derivatives of benzene. For example:

- Aliphatic hydrocarbons They are hydrocarbons that do not have aromatic character. They can be linear or cyclical. For example:
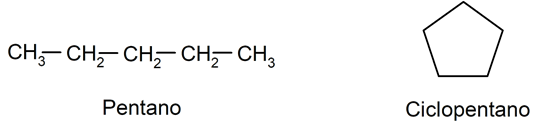
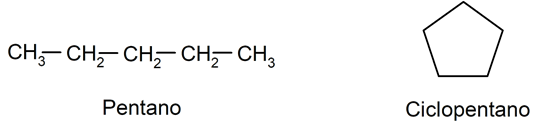
- Organometallic compounds They are organic compounds made up of carbon atoms covalently bonded to one or more atoms of a metallic element. For example:
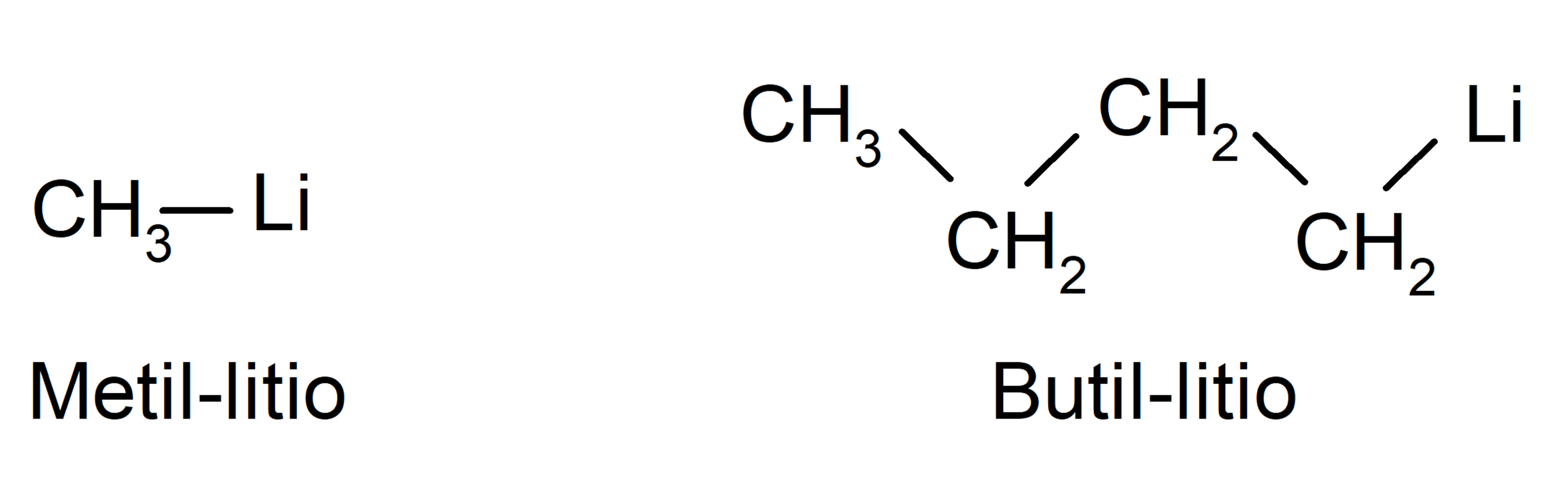
According to the functional groups they have (-OH, O=C, -NH2among others):
- Alkanes, alkenes and alkynes They are hydrocarbons that contain structures based on carbon and hydrogen, although they may also have other bonded atoms. In alkanes the carbon atoms are linked by single bonds, in alkenes by double bonds and in alkynes by triple bonds. For example:


- Alcohols. They are hydrocarbons that have a hydrogen replaced by a hydroxyl group (-OH). If several hydroxyl groups replace several hydrogens, they are called polyalcohols. For example:


- Ketones. They are organic compounds that have in their structure a carbonyl group (O=C=) linked to two carbon atoms. For example:
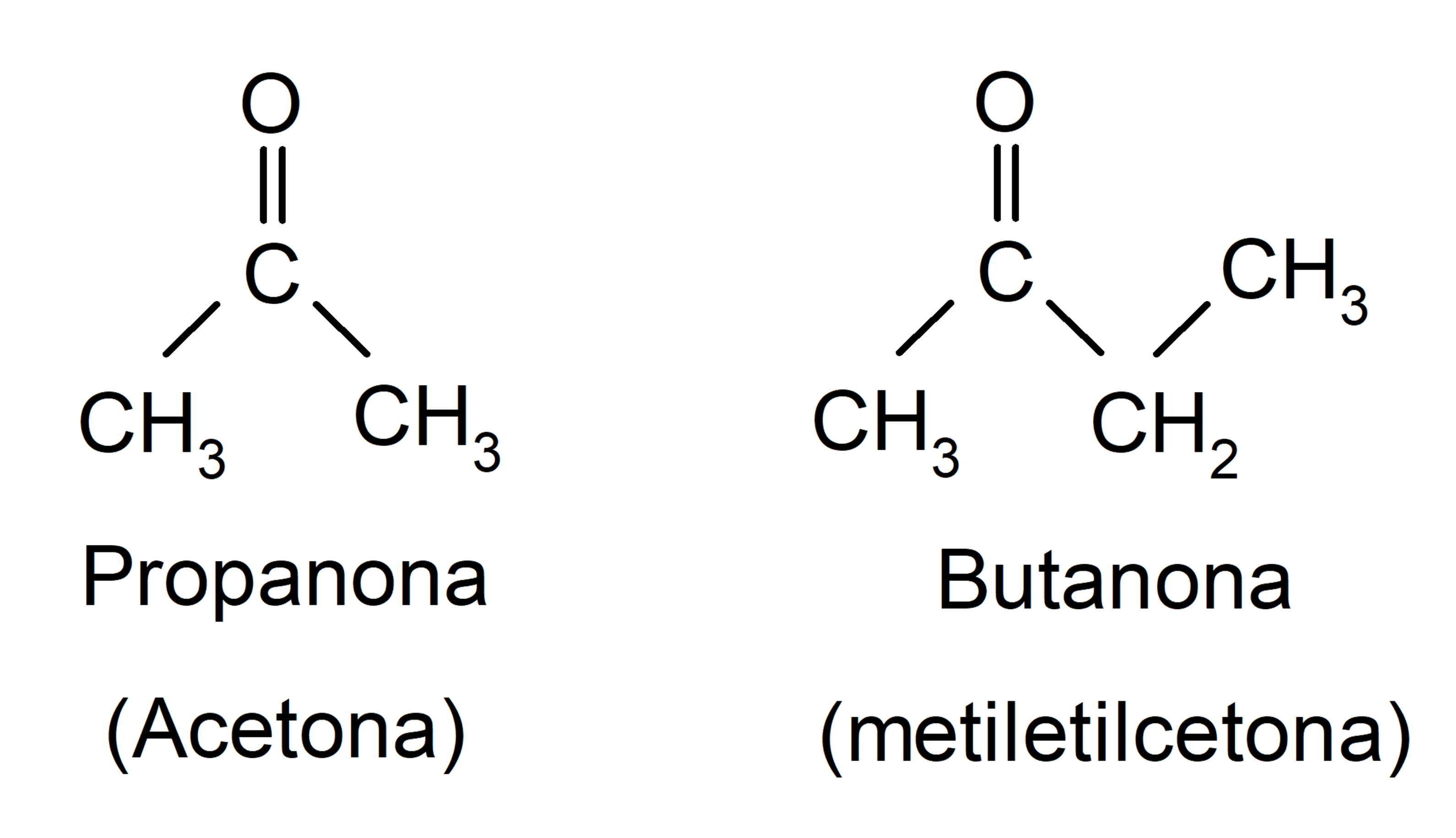

- Aldehydes. They are organic compounds that have in their structure a carbonyl group (O=C=) bonded to a hydrogen atom and a carbon atom. For example:
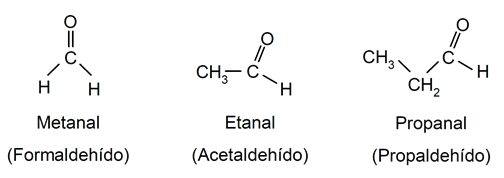
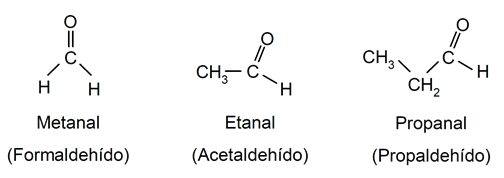
- Carboxylic acids. They are organic compounds that have a carboxyl group (-COOH) in their structure. For example:
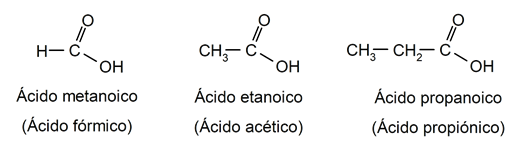
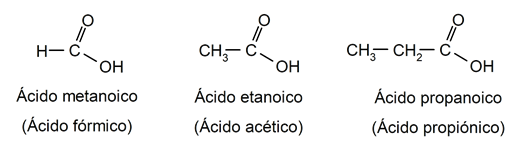
- Amines. They are organic compounds whose structure comes from replacing one or more hydrogens of the ammonia molecule (NH3), by certain substituents. For example:
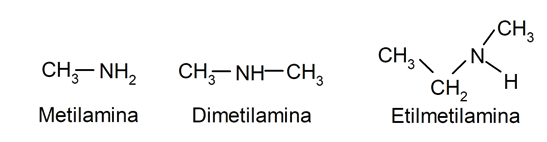
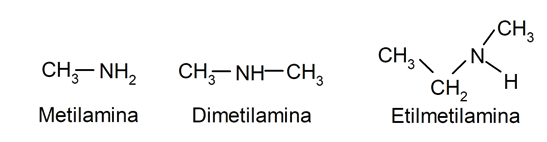
Depending on its size or molecular weight:
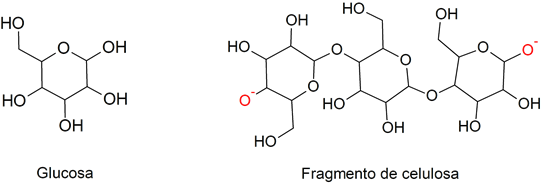
- Monomers They are molecular units that are linked by chemical bonds to form macromolecules called polymers. For example: glucose.
- Polymers. They are macromolecules composed of smaller molecular units called monomers. For example: cellulose.

Organic chemistry and inorganic chemistry
The essential difference between organic and inorganic chemistry has to do with the type of compounds in which they are interested. Organic chemistry studies compounds whose structure is based on carbon and hydrogen as main components.
Instead, Inorganic chemistry deals equally with the rest of the chemical elements capable of being part of the substances that sustain life, but not as fundamental and primordial elements. Therefore, there are inorganic compounds that contain carbon and hydrogen, but there are no organic compounds without carbon.
Thus, inorganic chemistry mostly explores compounds formed by bonds that involve electrostatic interactions, as well as metallic compounds, which for the most part are good conductors of heat and electricity. Instead, organic chemistry studies compounds formed by covalent bonds, which are bonds formed when electrons from the lower energy levels of atoms are shared.
Examples of organic chemistry

Organic chemistry is very present in our daily lives in chemical processes, both natural and artificial:
- The making of soap It is produced through the process called “saponification”, from the use of animal and vegetable fats.
- Fermentation and distillation of sugars It is carried out by microorganisms to obtain alcohols. With them, humans make drinks, solvents and various products.
- The synthesis of starches It is the process that plants carry out during their photosynthesis, and which helps them store carbohydrates in cotton and other similar materials, which can also be used by humans.
- The petrochemical industry. From petroleum, polymer chains are obtained that are used to manufacture substances as different as plastic, gasoline, benzene, etc.
- The creation of antibiotics. Certain fungi secrete these compounds capable of killing certain types of bacteria. In addition, there are antibiotics that are synthesized in laboratories.
Continue with: Chemical industry
References
- “Organic chemistry” https://es.wikipedia.org/
- “Organic chemistry: Introduction” (video) in Educatina. https://www.youtube.com/
- “Organic chemistry” https://es.khanacademy.org/
- “Organic Chemistry” https://www.britannica.com/





