We explain what oxidation is and how it occurs. Also, the types of oxidation, oxidation number and reduction.

What is oxidation?
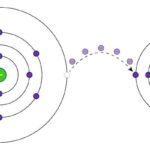
Oxidation is commonly called chemical reactions in which oxygen combines with other substances forming molecules called oxides. This is particularly common in the world of metals, although by no means exclusive to them. In chemistry, oxidation is the chemical phenomenon in which an atom, molecule or ion loses one or more electrons, thus increasing its positive charge.
As oxygen is an element that usually accepts these electrons, this type of reaction was called reduction-oxidation reactions, oxidation-reduction reactions or redox reactions, but it is also important to clarify that there may be redox reactions in which no oxygen participates. Let's take into account that the name oxygen comes from the Greek oxys“acid”; and genos“producer”: that is, oxygen is called that because it corrodes metals, just as acid does.
Most cases of oxidation involve oxygen, but it can also occur in its absence. And similarly, oxidation and reduction always occur together and simultaneously.
Two elements always participate in them and exchange electrons:
- The oxidizing agent. It is the chemical element that captures the transferred electrons, that is, it receives them and increases its negative charge. This is called having a lower oxidation state, or in other words, being reduced.
- The reducing agent It is the chemical element that gives up or loses the transferred electrons, increasing its positive charge. This is called having a higher oxidation state, or in other words, being oxidized.
Then: the oxidizing agent is reduced by the reducing agent, at the same time that the reducing agent is oxidized by the oxidizing agent. In this way, we have to to oxidize is to lose electrons while to reduce is to gain electrons.
These processes are common and everyday, in fact they are essential for life: living beings obtain chemical energy thanks to similar reactions, such as the oxidation of glucose.
See also: Oxidizer
Types of oxidation

There are two known types of oxidation:
- Slow oxidation. It is produced due to the oxygen contained in the air or water, which causes metals to lose their shine and suffer corrosion when exposed to the environment for too long.
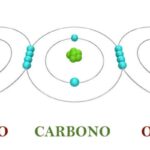
- Rapid oxidation. It occurs in violent chemical reactions such as combustion, generally exothermic (they release energy in the form of heat), and occurs mainly in organic elements (containing carbon and hydrogen).
Oxidation number

Chemical elements have an oxidation number, which represents the number of electrons that said element puts into play when associating with others to form a specific compound.
This number is almost always integer and can be positive or negative, depending on whether the element in question loses or gains electrons during the reaction, respectively.
For example: an element with oxidation number +1 tends to lose an electron when reacting with others, while one with oxidation number -1 tends to gain an electron when reacting with others to form a compound. These oxidation numbers can have values as high as electrons involved in the process and they usually depend in some cases on what elements they are reacting with.
Free elements, that is, those that are not combined with others, have an oxidation number of 0. On the other hand, some examples of oxidation numbers are:
The oxidation number of oxygen is -2 (O-2), except in peroxides that have -1 (O2-2) and in superoxides that have -½ (O2–).
The oxidation number of metallic elements is positive. For example: sodium ion (Na+), magnesium ion (Mg2+), iron ions (Fe2+Faith3+)
The oxidation number of hydrogen is +1(H+), except in metal hydrides that have -1 (H–).
Oxidation and reduction
Oxidation and reduction They are inverse and complementary processes which always occur at the same time. In the first, electrons are lost and in the second they are gained, thus varying the electrical charges of the elements.
These reactions are often used in industrial and metallurgical processes for example, to reduce minerals obtaining pure metallic elements such as iron or aluminum; or in the combustion of organic matter, such as in electricity generation plants or even in jet engines.