We explain what an oxidizer is, what reactions it is involved in and some examples. Fuel and activation energy.

What is an oxidizer?
An oxidizer or oxidant is a chemical substance or compound that has the property of being reduced that is, to gain electrons, when it is part of an electrochemical or oxidation-reduction reaction. These are chemical compounds that oxidize others with which they react, removing electrons from them.
In this type of reaction, known as redoxthe two processes occur simultaneously: the oxidation of one compound (the fuel) and the reduction of the other (the oxidizer). All the compounds involved have an oxidation state, and energy is usually released while the reaction occurs, that is, it is an exothermic reaction. The classic example of this type of reaction is combustion.
The best known oxidizer of all is oxygen essential in practically all forms of combustion, and present in the Earth's atmosphere in proportions of up to 21%. It is for this reason that we cannot light a fire without a minimum presence of air, since air is a mixture of oxygen and other gases.
See also: Chemical phenomena
Examples of oxidizer
Some known oxidizing or oxidizing agents are the following:
- Oxygen (O). It is the most common fuel on planet Earth. In fact, we use it in our body to oxidize glucose molecules in food and thus obtain chemical energy to stay alive.
- The bleaches. As hypochlorite (ClO–) and other hypohalites, as well as chlorites (ClO2–), chlorates (ClO3–) and similar halogen compounds.
- The hydrogen peroxide. Known as hydrogen peroxide (H2EITHER2).
- Permanganate salts. For example, potassium permanganate (KMnO4).
- The sulfoxides. For example, peroxosulfuric acid (H2SW5).
- Tollens reagent. An aqueous diamine-silver complex that is used in laboratories, precisely, as an oxidant.
- Most compounds containing Cerium (IV)
Oxidant and fuel
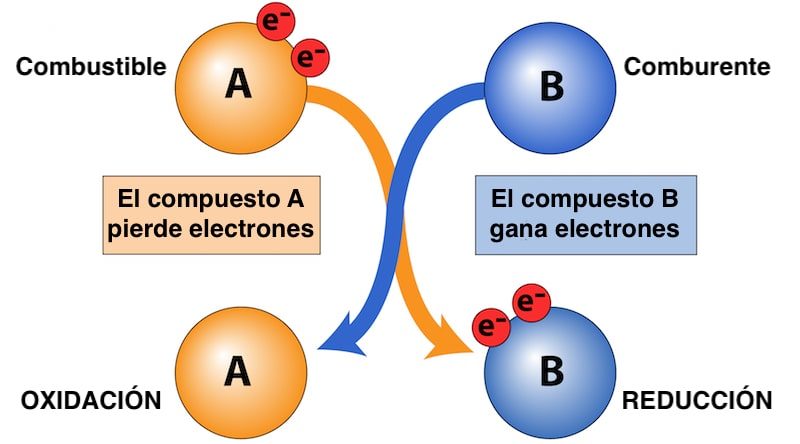
If the oxidizer is the compound that gains electrons during the redox reaction, fuel is the substance that gives up electrons and oxidizes unlike the oxidizer (which is reduced).
By doing so, the fuel releases part of its chemical energy as heat thus allowing, for example, combustion to occur. Both fuel and oxidizer are essential for this type of chemical reactions to occur.
Some typical fuels are coal, wood, hydrocarbons, gasoline, natural gas, etc.
Activation energy

The activation energy is a minimum initial energy charge that triggers the reaction. It is the last element necessary for combustion to occur, apart from fuel and oxidizer.
By themselves, fuel and oxidizer do not usually react but if we add an additional load of energy, in the form of heat, we will trigger combustion until the fuel has been consumed.
A clear example is lighting a bonfire. We have the fuel (the wood), the oxidizer (the oxygen in the air), but we need to light a match or match to start the combustion.
The same thing happens with a lighter: we have the fuel (liquefied gas), the oxidizer (the oxygen in the air) and we only require the additional energy of the spark, produced by the rotation of the wheel on the lighter.
Continue with: Chemical risk
References
- “Oxidant” on Wikipedia.
- “Comburent” in Enciclopedia.es.
- “Fuel, oxidizer and activation energy” in Expower.
- “Comburent” in Previpedia.
- “Fuel. Oxidant. Heat” in Construmática, Metaportal of Architecture, Engineering and Construction.