We explain what oxygen is, what its properties, its function and uses are. Also, its importance and its history.

What is oxygen?
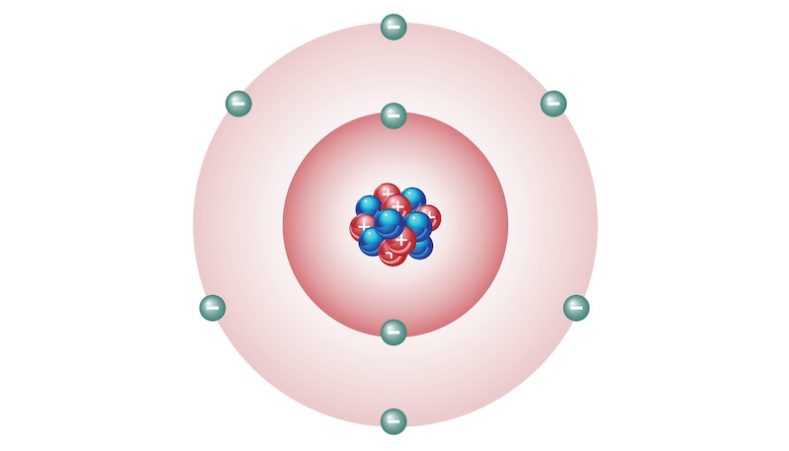
Oxygen is a chemical element of the Periodic Table that is represented by the symbol O. It is the most abundant element on Earth and the most abundant in the universe, after helium (He) and hydrogen (H).
It has atomic number 8 and atomic mass 15.9994 amu. It is a non-metal, it is very reactive and it has a very high electronegativity (ability of an atom to attract to itself the electrons of the chemical bond it forms with another atom), surpassed only by that of fluorine (F).
Under normal conditions of pressure and temperature (1 atm and 20 ⁰C) oxygen exists as molecular oxygen (O2), a diatomic gas (made up of two atoms) that is odorless, colorless and tasteless. Additionally, oxygen makes up approximately 21% of the air.


See also: Lithium
Properties of oxygen
Oxygen is a very reactive chemical element that, if subjected to different pressures and temperatures, can exist in different states of aggregation. Some of its physical and chemical properties are:
Physical properties of oxygen
- Under normal conditions of pressure and temperature it is an odorless, colorless and tasteless gas.
- It has a boiling point of -183 ºC and a melting point of -218.8 ºC.
- When it condenses, it transforms into a light blue liquid.
- It is soluble in water.
- It is heavier than air.
- It is paramagnetic, that is, it is magnetized by a magnetic field. This means that it is attracted to a magnet, although if the magnet is removed, the oxygen is demagnetized.
Chemical properties of oxygen
- It has an oxidation state of -2 in almost all the chemical compounds it forms, although when it forms peroxides (O22-) has oxidation state -1 and when it forms superoxides (O2–) has oxidation state -½.
- At high temperatures, it forms oxides with almost all chemical elements.
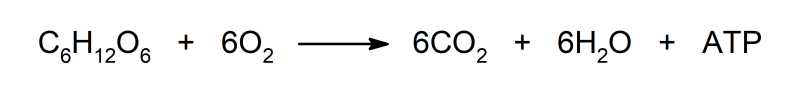
- It reacts with organic compounds, such as glucose, oxidizing them and producing carbon dioxide (CO2), water (H2O) and releasing energy in the form of ATP (adenosine triphosphate).
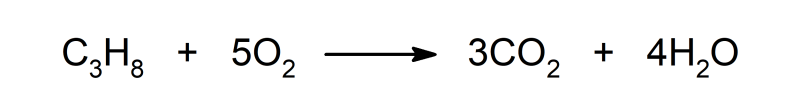
- It produces combustion reactions with organic compounds such as propane, in which carbon dioxide (CO) is produced.2), water (H2O) and energy is released in the form of light and heat.
- It produces corrosion of metals such as iron (Fe), and thus forms oxides.
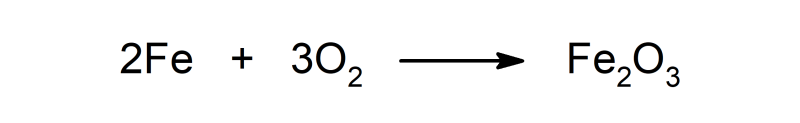
Inorganic oxygen compounds
Inorganic compounds are chemical compounds that do not have their main structure based on carbon, although they may contain carbon. Oxygen is part of many inorganic compounds and among these, some are fundamental chemical compounds to sustain life on the planet. This is the case of water (H2O) and carbon dioxide (CO2).
Some inorganic chemical compounds that contain oxygen are:
The water (H2EITHER)
Water is one of the most important chemical compounds to maintain life on the planet. It makes up approximately 71% of the Earth's crust.
Carbon dioxide (CO2)
Carbon dioxide is one of the fundamental chemical compounds for plants to carry out photosynthesis. In addition, it is a component that causes the greenhouse effect, so it is important to reduce the emission of this compound into the atmosphere.
metal oxides
Metal oxides are compounds formed by oxygen and a metal. At high temperatures a large amount of oxides are formed, although at room temperature (25 ºC) oxides are also formed. For example:
- Iron(III) oxide (Faith2EITHER3). It is the rust found on structures made of steel or iron.
- Iron(II) oxide (Ugly). It is an explosive compound, so it must be handled with caution.
- aluminum oxide (To the2EITHER3). It is one of the main components of clays and glazes. It is used as a thermal and electrical insulator.
- magnesium oxide (MgO). It is an oxide that is used in medicine, in the production of cement for construction and is part of fertilizers.
non-metallic oxides
Nonmetal oxides are compounds formed by oxygen and a nonmetal. For example:
- Nitrogen monoxide (NO) and nitrogen dioxide (NO2). They are atmospheric pollutants that contribute to the formation of the ozone layer hole.
- sulfur dioxide (SW2) and sulfur trioxide (SW3). At room temperature they are gases. They are pollutants and constitute the main components of acid rain.
- Chlorine(VII) oxide or dichloroheptoxide (Cl2EITHER7). It is an oily, volatile liquid that explodes when impacted or comes into contact with fire.
The oxysalts
Oxysalts are salts that contain oxygen. When dissolved in water, the resulting solution conducts electricity. For example:
- zinc sulphate (ZnSO4). It is used in medicine as a dietary supplement.
- sodium carbonate (Na2CO3). It is used in the production of soaps and glass.
- He potassium nitrate (KNO3). It is used to produce fertilizers and in food preservation.
Organic oxygen compounds
Organic compounds are chemical compounds that have their main structure based on carbon, in addition, they are the main body builders of living organisms. Oxygen is part of many organic compounds, which makes it a fundamental chemical element to sustain life on the planet.
Some organic chemical compounds that contain oxygen are:
The alcohols
Alcohols are organic compounds that contain a hydroxyl functional group (-OH), formed by oxygen (O) and hydrogen (H). For example:
- He methanol (CH3OH). It is used as vehicle antifreeze and a solvent for many substances.
- He ethanol (CH3CH2OH). It is used to produce alcoholic beverages, as a solvent and as a disinfectant. In addition, it is used as fuel.
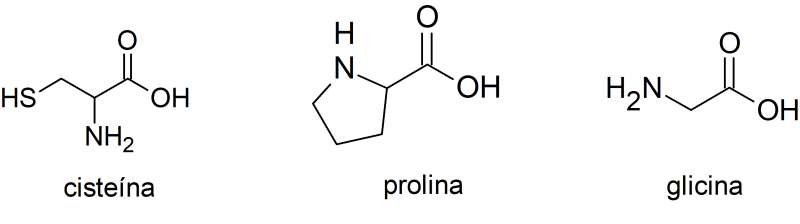
The amino acids
Amino acids are molecules that constitute the units that make up proteins. For example:
- Cysteine. It is used in the pharmaceutical and food industries. It is mainly found in raw meat, breast milk and some vegetables.
- The proline. It is used in the treatment of diseases such as arthritis, tendinitis and torticollis. It is found in collagen.
- The wisteria. It is used to develop and recover muscles after physical exertion.
organic acids
Organic acids are organic compounds that have the carboxyl functional group (-COOH), formed by carbon (C), oxygen (O) and hydrogen (H). They do not dissolve completely in water and are less reactive with metals than inorganic acids. For example:
- He methanoic acid or formic acid (HCOOH). It is the acid that some ants inject when they bite. It is a component of honey.
- He ethanoic acid or acetic acid (CH3COOH). It is the acid responsible for the smell and taste of vinegar.
What is oxygen used for?
Oxygen is a very important element: life would not be possible without oxygen but, in addition, human beings have found ways to use it at industrial levels, for the manufacture of products used in many sectors, both metallurgical, cosmetics, and medicine.
Biological importance of oxygen
Oxygen is essential for maintaining the life of living beings. The process of cellular respiration, by which living organisms produce the energy necessary to live, takes place in the presence of oxygen. Without this element, living beings would not be able to breathe and life on the planet would end.
Thanks to the constant intake of oxygen to living organisms, it is possible for them to carry out their vital functions such as respiration, digestion, growth and reproduction. Additionally, oxygen allows these organisms to have enough energy to move from one place to another.
Industrial uses of oxygen
Oxygen is used in various industrial sectors and is essential in many processes, vehicles and equipment such as:
- Respiratory assistance equipment.
- Water disinfection processes.
- Glass manufacturing.
- Smelting in furnaces for the metallurgical industry.
- Processes that involve controlled oxidation of chemical compounds.
- Conservation and treatment of food.
- Activities and techniques to raise aquatic species.
- Oxygenate interiors of airplanes, spaceships and submarines.
Where is oxygen found?

Oxygen is found in gaseous form in the atmosphere as molecular oxygen (O2), and forms approximately 21% of the air. In addition, the water of rivers, seas and lakes contain dissolved oxygen.
On the other hand, oxygen is found as part of some fundamental molecules to sustain life on the planet, such as water (H2O), carbon dioxide (CO2), the amino acids that make up proteins and DNA. Oxygen is part of living organisms.
Oxygen also forms ozone (O3) in the ozone layer, and at altitudes above the Earth's low orbit, it is found in the form of atomic oxygen (O).
Environmental impact of alterations in oxygen levels
The decrease in oxygen on the planet can cause severe damage to living organisms, or even death. A current problem is the use of fertilizers, which run off into lakes and rivers, causing an excess of nutrients that generates an overpopulation of microorganisms and algae that consume a lot of dissolved oxygen.
This process is called eutrophication and causes a decrease in dissolved oxygen, which in turn causes the death of fish and other aquatic animals that do not have enough oxygen to live. On the other hand, the increase in water temperature has also caused a decrease in dissolved oxygen, which leads to the death of aquatic life.
Discovery and history of oxygen
In 1772, the pharmacist Carl Wilhelm Scheele managed to produce gaseous oxygen from the heating of mercury (II) oxide (HgO) and some nitrates. He called this oxygen produced “fire air.” However, this discovery was published in 1777.
On the other hand, in 1774 Joseph Priestley caused sunlight to hit mercury (II) oxide (HgO) that was contained in a glass tube, and he could see that a gas began to appear inside the tube. He also noticed that if he placed a mouse and candles in contact with that gas, the mouse became more active and the candles burned brighter. Then, in 1775, he published the result of his experiments in the article “Report of Further Discoveries in the Air.” It is Joseph Priestley who is considered the discoverer of oxygen, as he first published his discoveries.
On the other hand, the French Antoine Lavoisier (chemist, biologist and economist) used the studies that began in 1774 to explain the combustion process and also carried out the first laboratory tests on oxidation.
Then, in 1777, Lavoisier published a book called “About combustion in general”, in which he shows that air is a mixture of two gases and called them 1) “essential air” (because it is the part of the air that is used for combustion and respiration) and 2) the “scourge”, which is the remaining part of the air. He later named this “essential air” oxygen.
oxygen cycle
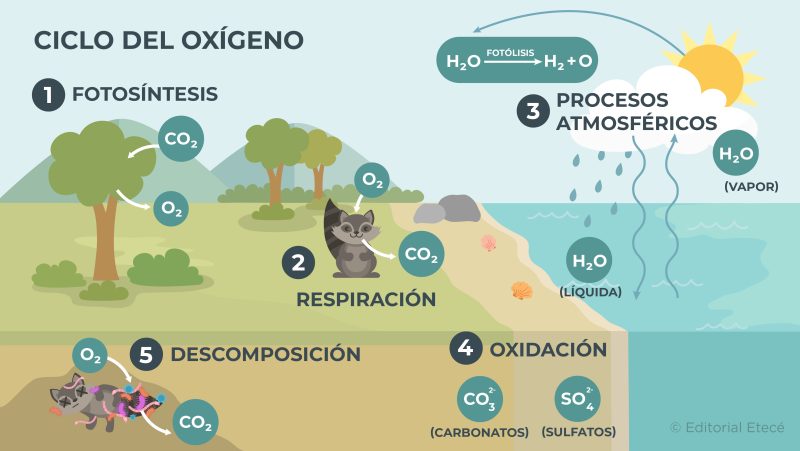
The oxygen cycle is the circulation of oxygen between the different ecosystems of the planet and the environment that surrounds them. Additionally, this cycle has several stages:
Rapid or biological stage
This stage involves the biological processes that occur in living beings. For example:
- Photosynthesis It is the process by which plants, algae and phytoplankton use water, carbon dioxide (CO2) and sunlight to produce the necessary nutrients that allow them to obtain energy to live. On the other hand, when these organisms carry out photosynthesis, they expel oxygen (O2) to the atmosphere.
- Breathing It is the process by which many living organisms use oxygen to convert certain nutrients into energy. When these organisms perform respiration, they expel carbon dioxide (CO2) and water vapor (H2O(g)) to the atmosphere.
Slow or geological stage
This stage involves the oxidation processes of some chemical elements and the decomposition of certain chemical compounds. For example:
- Part of the hydrological cycle and atmospheric processes During the hydrological cycle, evaporation of water from the earth's surface occurs. Then, the water molecules present in the atmosphere interact with solar radiation, which separates them into oxygen and hydrogen.
- Oxidation. When oxidation of chemical elements occurs, chemical compounds are formed that contain oxygen and can then decompose and release the oxygen.
- Decomposition When organisms die, they decompose due to the action of certain microorganisms that use oxygen to carry out the decomposition process and then release CO2.
- oxygen cycle
- Biogeochemical cycles
- Periodic table
References
- Torres, W. H. (2002). Biology of reactive oxygen species. Biochemical message, 2619-54.
- Mora Orozco, CDL, Flores López, HE, Durán Chávez, Á., & Ruiz Corral, JA (2011). Climate change and the impact on the concentration of dissolved oxygen in Lake Chapala. Mexican journal of agricultural sciences, 2(SPE2), 381-394.
- Djerassi, C., & Hoffman, R. (2014). Oxygen: play in 2 acts. Economic Culture Fund.
- Chacón Sánchez, LA (2019). oxygen.