We explain what polymers are, their classification, properties and characteristics. In addition, natural and synthetic polymers.
What is a polymer?
In chemistry, polymers are a type of macromolecules made up of chains of simpler units called monomers, linked together by covalent bonds. Its name comes from Greek polys (“many”) and groupers (“segment”).
They are generally organic molecules of enormous importance in both the natural and industrial world. These molecules include the DNA in our cells, starch in plants, nylon, and most plastics.
At the end of the 19th century and the beginning of the 20th, it was discovered how to manipulate them. Thus, the handling of materials by humanity was revolutionized forever.
- If classified according to their origin, polymers can be:
- Natural polymers Its origin is biological.
- Synthetic polymers They are created entirely by humans.
- Semi-synthetic polymers They are created by transformation of natural polymers.
- If they are classified according to their composition, we can distinguish between:
- Organic polymers They have a main chain of carbon atoms.
- Vinyl organic polymers. Similar to organic ones, but with carbon-carbon double bonds. They include polyolefins, styrenics, halogenated vinyls and acrylics.
- Non-vinyl organic polymers. They have oxygen and/or nitrogen atoms in their main chain, in addition to carbons. They include polyesters, polyamides and polyurethanes.
- Inorganic polymers. Based on other elements such as sulfur (polysulfides) or silicon (silicone).
- If they are classified according to their reaction to increasing temperature, we can distinguish between:
- Elastomeric polymers They deform as the temperature increases, but recover their original shape.
- Thermostable polymers When they rise, their temperature decomposes chemically. They do not deform, that is, the material does not flow.
- Thermoplastic polymers When the temperature is raised, they melt and become liquid, but when they cool, they become solid again.
Natural polymers
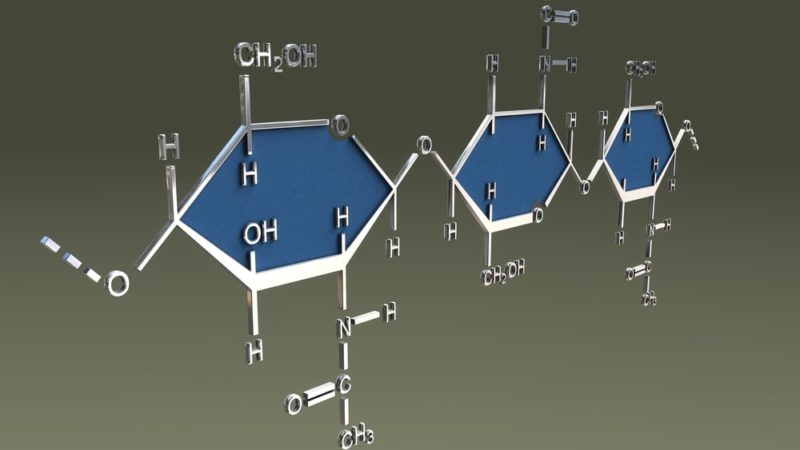
Natural polymers exist as such in nature such as biomolecules and compounds that make up the body of living beings. The appearance of natural polymers in the world represented an important point in the biochemical complexity of life.
These include the vast majority of proteins, nucleic acids, polysaccharides (complex sugars, such as plant cellulose and fungal chitin), and vegetable rubber.
Synthetic polymers

The first synthetic polymer was created in 1907: Bakelite durable and low-cost material. Its great industrial success was largely due to its simple and economical manufacture, using phenol and formaldehyde. Much progress has been made since then in obtaining new and more powerful materials of organic origin, particularly in the petrochemical industry.
The polymers can created in the laboratory by joining specific monomers into a chain using organic or inorganic inputs, under controlled conditions of temperature, pressure and presence of catalysts. Thus, a chain or step reaction is generated that results in the generation of the compound.
Properties and characteristics of polymers
In general terms, polymers are bad electrical conductors which is why they are often used as insulators in the electrical industry, for example, plastic as wrapping cables. However, there are conductive polymers, created in 1974, whose applications are still being studied today.
Temperature, on the other hand, is an important factor in the behavior of polymers. At low temperatures they become hard, brittle similar to glass, while at normal temperatures they tend to be elastic. If the temperature increases towards their melting point, some begin to lose their shape and others may decompose.
Examples of polymers

Some of the best-known polymers and of greatest human importance are:
- Polyvinyl chloride Also known as PVC and with the general formula (C2h3Cl)n, is obtained from the polymerization of vinyl chloride units. It is the most versatile plastic derivative known and is used for all types of packaging, footwear, coatings, flexibles and even pipes.
- Polystyrene Known as PS, it is obtained from styrene monomers, and can obtain very diverse results: more or less transparent, more or less brittle, or even very dense and waterproof variants. It was first synthesized in Germany in 1930 and since then about 10.6 million tons have been produced annually in the world.
- Polymethylmethacrylate Abbreviated with the acronym PMMA, it is a typical engineering plastic, and it is one of the most competitive in terms of its industrial applications, since it is extremely transparent and resistant.
- Polypropylene Referred to in its acronyms as PP, it is a thermoplastic polymer, partially crystalline and made from propylene or propene. It is used in food packaging, fabrics, laboratory equipment and transparent films or films to cover objects.
- Polyurethane These polymers are obtained by combining hydroxyl bases and diisocyanates, and can be thermoplastic or thermostable. They are frequently used in the footwear industry, paint, synthetic textile fibers, packaging, condoms or components of machines and vehicles.
Continue with: Polyethylene
References
- “Polymer” on Wikipedia.
- “The polymers. Concept.” (video) at the University of Burgos.
- “Polymers” in Science Magazine of the National Autonomous University of Mexico.
- “Polymers: Crash Course Chemistry #45” (video) in Crash Course.
- “What is a Polymer” on LiveScience.
- “Polymer (chemistry)” in The Encyclopaedia Britannica.





