We explain what a product is in chemistry, the process by which a product is obtained and how to calculate the yield of a reaction.

What is a product in chemistry?
In chemistry and its branches, the substances obtained after a chemical reaction occurs.
A chemical reaction involves two or more substances (simple or compound), called reactants either reagentsand that contribute to the reaction the atomic or molecular material that will be altered or modified during the reaction.
Once the chemical bonds of the reactants have been modified, and a certain amount of energy has been generated or consumed when the reaction occurs, we will have one or more products available.
The products obtained from certain types of reagents will depend directly on the conditions under which the chemical reaction occurs and the nature of the reagents. Conditions such as temperature or presence of catalysts (other substances that affect the speed of the reaction) are determining factors in the time it takes for a reaction to occur.
However, whatever the chemical reaction considered and the conditions under which it occurs, the amount of matter and energy must be conserved, that is, The number of reactants (atoms, molecules, ions) that react must be equal to the number of products formed and the energy involved at the beginning of the reaction must be equal to the energy involved at the end of the reaction, whether this energy is consumed or released in any of the stages of the reaction.
It is very important to understand that during a chemical reaction the amount of matter and energy of the reactants is neither created nor destroyed to become products, it is only transformed into them.
See also: Exothermic reaction
Reaction yield
In the same way, the quantities formed (real) of product are never usually identical to those considered theoretically since this is influenced by specific properties such as the purity of the reactants or the secondary reactions that occur, as well as the environmental conditions in which the reaction is generated, for example, temperature and humidity.
The actual product quantities (those obtained in practice and not as a result of a theoretical calculation) are lower than the theoretical ones because, due to the above reasons, product can be lost in purification steps subsequent to the reaction, in secondary reactions where these products intervene or in evaporations if they are volatile.
The maximum amount of product that can be obtained during a chemical reaction is called the theoretical yield. To calculate the theoretical yield it is necessary to know the limiting reactant in the reaction (reagent that is used up first during the reaction).
The amount of actual product obtained in a chemical reaction is called the percent yield.
In the following example we will see how to calculate the theoretical yield and the percentage yield of a chemical reaction, in which the limiting reactant will have to be identified.
Suppose we have the following reaction where 2.80 g of aluminum react with 4.25 g of dichlorine:
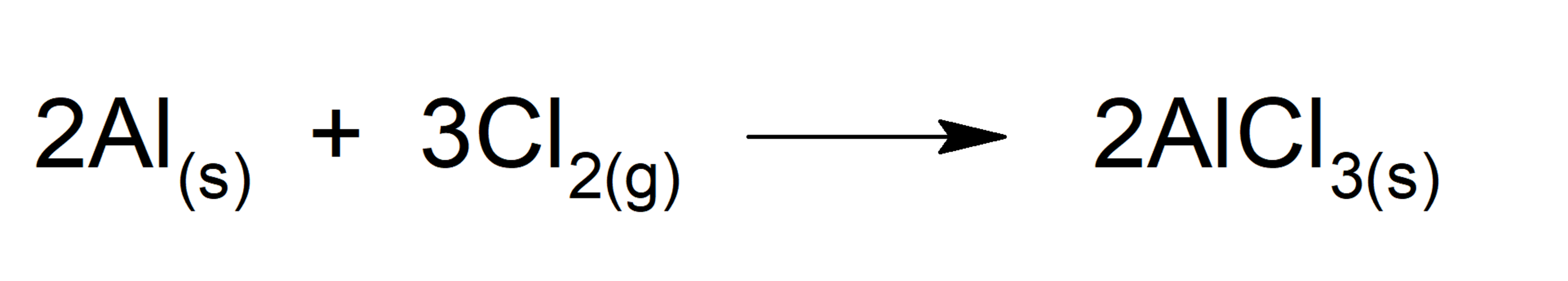
There are different methods to identify the limiting reagent and it is important to clarify that the limiting reagent is not necessarily the reagent that has the greatest mass when the reaction begins. We will describe two of these methods:
Method 1 It consists of calculating the number of real moles of the reactants using the real masses and the molar masses (Evil) and M(Cl2 ) in this case) of each reagent. Then, the actual molar relationship (quotient between quantities of substances (moles)) between the reactants is calculated, that is, using the initial masses. This actual molar ratio is then compared to the stoichiometric ratio of the reactants in the balanced equation (calculated using the stoichiometric coefficients).
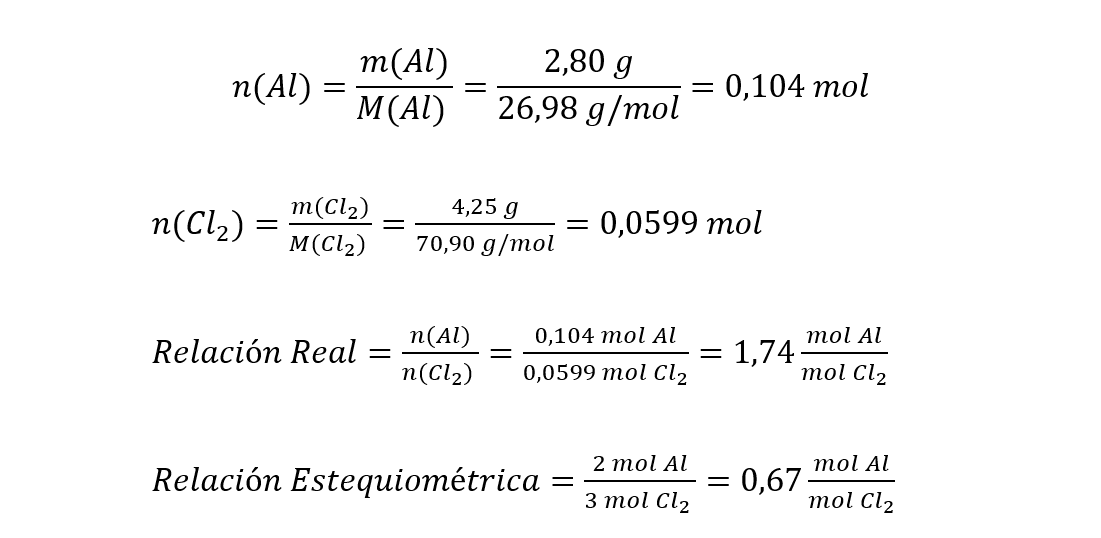
It can be seen that the real ratio is greater than the stoichiometric ratio, so aluminum (whose number of moles are located in the numerator of the quotient) is in excess and dichlor is the limiting reactant.
Method 2. In this method we use the definition of mole of reaction. One mole of reaction is obtained when the stoichiometric coefficients of the adjusted or balanced chemical equation react. In the reaction we are analyzing, 1 mole of reaction is obtained when 2 moles of aluminum react with 3 moles of dichlorine to produce 2 moles of AlCl3which can be represented in the following equations:

In this way, a reaction occurs more times the more moles of reaction it has. The reagent with the smallest number of moles of reaction is the limiting one, since with this reagent the reaction can occur fewer times.
Using the moles of reaction and the moles of reactant, the limiting reactant can be identified as follows:
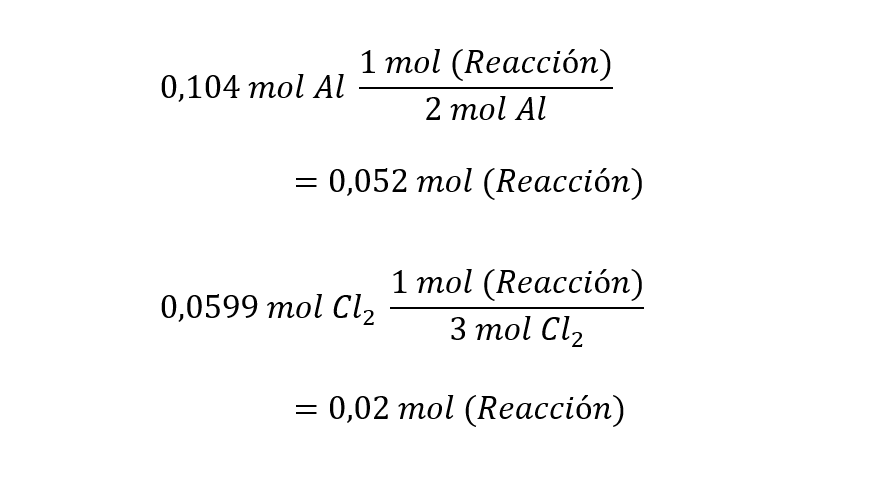
According to this method, the Cl2 It is also the limiting reagent, since it generates fewer moles of reaction.
Once we know that dichlor is the limiting reactant, we can calculate the theoretical yield as:
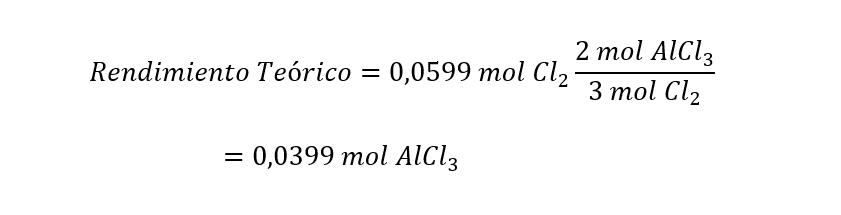
Next, we convert the moles of the theoretical yield to grams, using the molar mass of AlCl3 (M(AlCl3 )), and then calculate the percentage return:
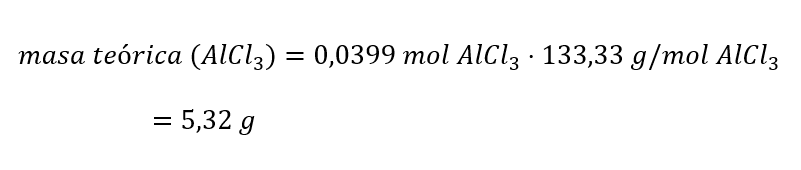
Finally, the percentage or actual yield of a chemical reaction is calculated:

And for the example we are analyzing it would be:

References
- «Limiting reagents and percentage yield» https://es.khanacademy.org/