We explain what hydrocarbons are, their characteristics and how they are classified. In addition, its derivatives, applications and environmental impact.

What are hydrocarbons?
Hydrocarbons are various types of organic compounds. They may have greater or lesser complexity, but they are always made up of a skeleton of carbon (C) and hydrogen (H) atoms as well as other possible elements.
Each hydrocarbon presents its structural patterns, since its specific configuration determines its physical and chemical properties, as well as the name of the substance in question. Oil, natural gas and coal are the main sources of hydrocarbons.
Most hydrocarbons are found inside the Earth buried beneath layers and layers of rock and soil. They are the product of the anaerobic decomposition, under very specific conditions, of large quantities of organic matter, which in ancient times constituted the body of different living beings.
The hydrocarbons are also present in the body of different living beings in specific forms such as the rubber generated by rubber trees, or a set of pigments called carotenes, which some vegetables have. Furthermore, they can be synthesized in a laboratory, with the appropriate raw materials.
Given their enormous chemical and energy potential, hydrocarbons are an indispensable part of various industries, including the production of electrical energy.
Characteristics of hydrocarbons
Some characteristics of hydrocarbons are:
- are made up of carbon and hydrogen mostly and possible additives of other elements or other radical groups. While its carbon atoms make up the structure of the compound, hydrogen atoms in some cases serve as a bridge to keep them together in a certain configuration (shape, structure, orientation).
- can have a linear or branched framework of molecules open or closed. Whether it is a hydrocarbon or another depends on its arrangement and quantity of components.
- They are highly flammable and have a huge energy capacity which makes them an ideal raw material for industrial transformation and for obtaining energy.
- Most of them are toxic and may give off vapors that are dangerous to health.
Classification of hydrocarbons
Since their discovery in the 19th century, hydrocarbons have been classified according to two possible criteria: its type of structure and the types of bond between its atoms. According to the first classification, two categories are distinguished:
- Acyclic or open chain hydrocarbons They are those whose chain of molecules does not close on itself. In turn, they can be divided into linear (line-shaped) or branched (with various branches). For example:
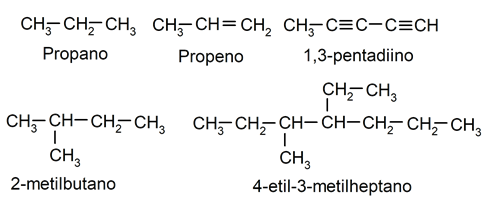
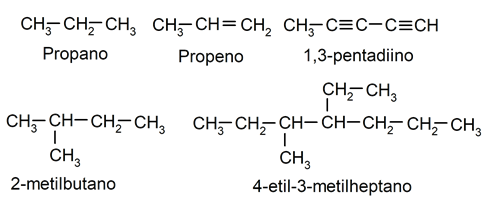
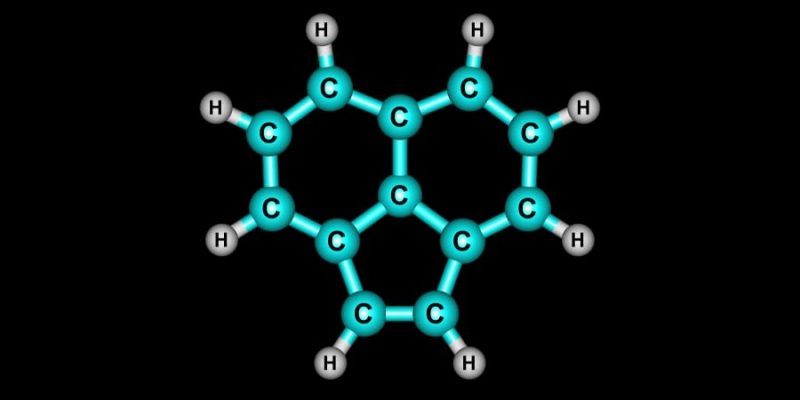
- Cyclic or closed chain hydrocarbons. They are those whose chain of molecules closes on itself. In turn, they can be divided into monocyclic (single cycle) and polycyclic (multiple cycles). For example:


Obeying the second classification, however, we have:
- Aromatic hydrocarbons. They have an aromatic ring, that is, a cyclic structure that obeys the so-called Hückel rule, which states that the number of delocalized electrons in an aromatic compound complies with:
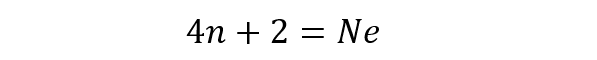
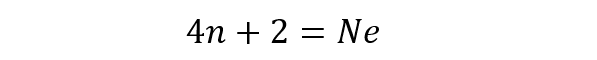
Where:
n. Represents an integer.
Ne. It represents the number of electrons delocalized in the aromatic compound.
For example, benzene (C6h6) has 6 delocalized electrons (called pi (𝛑) electrons) in its structure, which implies that n must be equal to 1.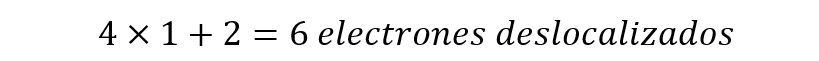
 almost all Aromatic hydrocarbons are usually derived from benzene (although not always) and, therefore, the hexagonal structure of benzene is part of many of these aromatic compounds. The name “aromatic” comes from the fact that these compounds were initially obtained by breaking down pleasant-smelling chemicals. Some examples of aromatic compounds are:
almost all Aromatic hydrocarbons are usually derived from benzene (although not always) and, therefore, the hexagonal structure of benzene is part of many of these aromatic compounds. The name “aromatic” comes from the fact that these compounds were initially obtained by breaking down pleasant-smelling chemicals. Some examples of aromatic compounds are: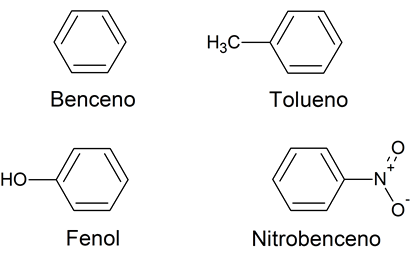

- Aliphatic hydrocarbons They lack an aromatic ring. Its name comes from Greek aleipharthat is, “fat”, since they were obtained from the decomposition of oils and fats. They are classified as saturated (with single atomic bonds) and unsaturated (with at least one multiple, double or triple bond). Some examples of aliphatic hydrocarbons are:
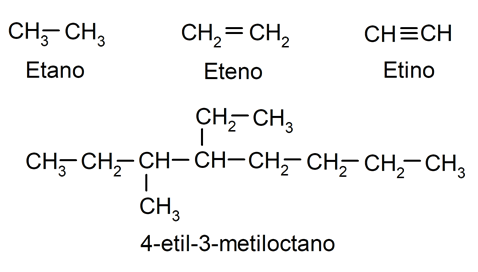
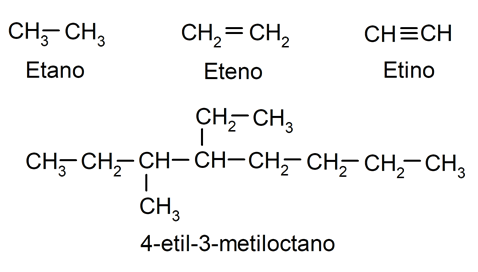
Importance of hydrocarbons
Hydrocarbons are tremendously versatile substances since they serve as raw materials to obtain very varied products. In addition, they have a great energy potential, that is, their combustion is easy and intense, so that they energetically support a diverse set of industries, ranging from materials, solvents, fossil fuels, to the generation of electrical energy.
Added to that is the fact that its formation took millions of years of slow chemical processes underground, so constitute an important but non-renewable resource which one day will be out of print forever (or at least for a good amount of time). Its use should occur under conditions of extreme responsibility.
Derivatives and applications of hydrocarbons

Hydrocarbons have a huge set of uses for humans, among which are:
- Energy generation Thanks to their enormous combustion capacity, hydrocarbons are used as a source of energy to generate electricity. This is carried out in certain types of power plants, and supplies energy to both homes and other industries and allows the support of our model of life.
- The generation of fuels Their energy capacity allows them to be used to manufacture various types of fuel (gasoline, diesel, liquefied natural gas), to power various types of vehicles, or to power various household appliances, such as heaters, stoves and heaters that operate using gases such as butane or propane.
- Obtaining plastics. Different types of plastic and versatile materials can be obtained in laboratories from the handling of hydrocarbons. These materials are so cheap, effective and easy to manufacture that there is a gigantic industry around them.
- The manufacture of solvents and other products. Many hydrocarbons are essential components of solvents and thinners, cleaning products, fertilizers, or bitumen.
Environmental impact of hydrocarbons
The use of hydrocarbons has its cost, and the first impact is on the environment. In general, these are toxic substances capable of causing great ecological damage in the event that they are discharged into nature, as occurs with oil spills or crude oil leaks. Repairing these environmental damages is usually expensive and time-consuming.
Furthermore, depending on the hydrocarbon, its release or its combustion emit greenhouse gases into the atmosphere that is, carbon-rich gases such as methane (CH4) or carbon dioxide (CO2).
These gases can destroy the planet's ozone layer, and also block the escape of energy through the atmosphere, contributing dramatically to global warming and climate change. The burning of fossil fuels is, in fact, one of the main factors in this global problem.
Continue with: Alternative energies
References
- “Hydrocarbon” https://es.wikipedia.org/
- “Importance of hydrocarbons” by Paz amría de Lourdes Cornejo at the Autonomous University of the State of Hidalgo (Mexico). https://www.uaeh.edu.mx/
- “Hydrocarbons” in Conicet Mendoza (Argentina). https://www.mendoza.conicet.gov.ar/
- “Hydrocarbon Overview” (video) on Khan Academy. https://www.youtube.com/
- “The origin of hydrocarbons” (video) at the Argentine Institute of Petroleum and Gas. https://www.youtube.com/
- “Hydrocarbon” https://www.britannica.com/





