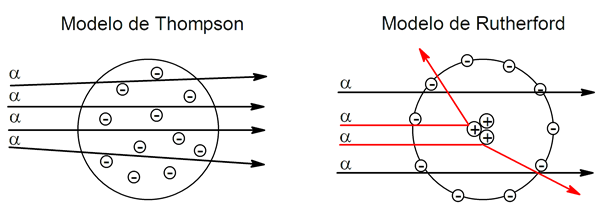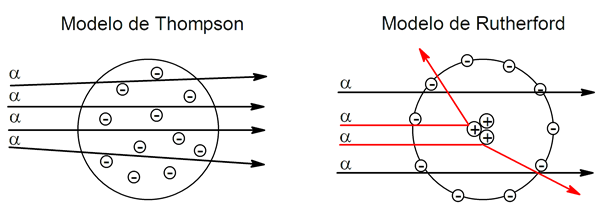We explain what Rutherford's atomic model is and its main postulates. Also, what was Rutherford's experiment like?
What is Rutherford's atomic model?
Rutherford's atomic model, as its name indicates, was one of the models proposed to explain the internal structure of the atom. In 1911, the British chemist and physicist Ernest Rutherford proposed this model based on the results of his experimentation with gold sheets.
This model constituted a break with previous models such as the Dalton atomic model and the Thompson atomic model, and a step forward with respect to the currently accepted model.
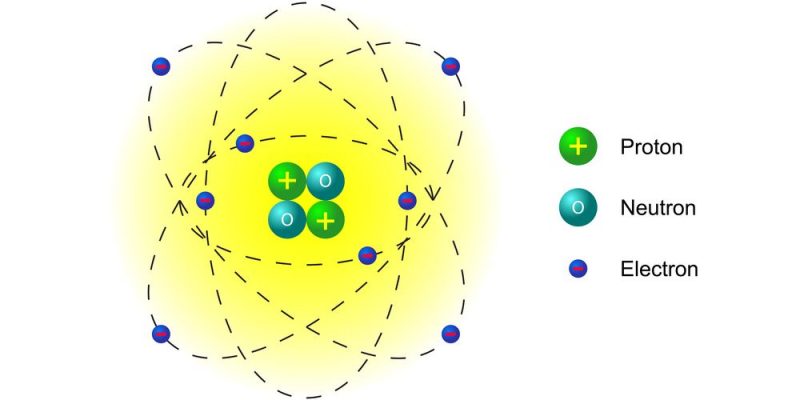
In his atomic model, Rutherford proposed that Atoms have a central nucleus where the largest percentage of their mass is found. Furthermore, according to this theory, this nucleus has a positive electrical charge and is orbited by particles of opposite charge and smaller size (electrons).
According to his considerations, the atom operated as a Solar System of electrons orbiting around a heavier atomic nucleus, as do the planets around the Sun.
Rutherford's atomic model can be summarized in the following three propositions:
- Most of the atomic mass is concentrated in the nucleus, larger and heavier than the rest of the particles, and endowed with a positive electrical charge.
- Around the nucleus and at great distances from it are electrons, with a negative electrical charge, which orbit it in circular paths.
- The sum of the positive and negative electrical charges of an atom should be zero, that is, they should be equal, for the atom to be electrically neutral.
Rutherford not only proposed this structure for the atom, but also calculated its size and compared it with the size of the nucleus, and concluded that a good part of the composition of the atom is empty space.
This model, on the other hand, has certain limitations that could be resolved with the advancement of knowledge and technology:
- It could not be explained how it was possible for a set of positive charges to be held together in the atomic nucleus, since they would have to repel each other, since they are all charges of the same sign.
- The stability of the atom could not be explained, because when considering the negatively charged electrons that revolve around the positive nucleus, at some point these electrons had to lose energy and collapse against the nucleus.
Rutherford's atomic model was in force for a short time, and was replaced by the atomic model proposed by the Danish physicist Niels Bohr in 1913, in which some of the limitations were resolved and the theoretical proposals developed by Albert Einstein in 1905 were incorporated.
See also: Proton
Rutherford's experiment
Rutherford's experimental method It started from several thin sheets of gold that would be bombarded in the laboratory with helium nuclei (alpha particles, which have a positive charge), thus measuring the angles of deflection of the particle beam as it passes through the gold.
This behavior, which sometimes reached deviations of up to 90°, did not agree with the atomic model proposed by Thompson, prevailing at the time.
Thompson's model proposes that the atom is a positive sphere with negatively charged electrons embedded in it. For this reason the model is similar to a pudding with raisins: the pudding would be the atom and the raisins would be the electrons.
On the other hand, Rutherford's model establishes that the atom has the positive charge concentrated in the nucleus and the electrons orbit around it. If the atom had the structure proposed by Thompson, the alpha (positive) particles, when passing through the gold foil, should follow their trajectories or deviate very little. However, what happened is that deviations of these particles of up to 90 and 180° were seen, which demonstrated that the atom, indeed, has the positive charge concentrated in its center (as Rutherford proposed) and not distributed in a sphere. (as Thompson proposed).