We explain what the periodic table is and what its history is. Also, how it is organized and what are the different groups it contains.
What is the Periodic Table?
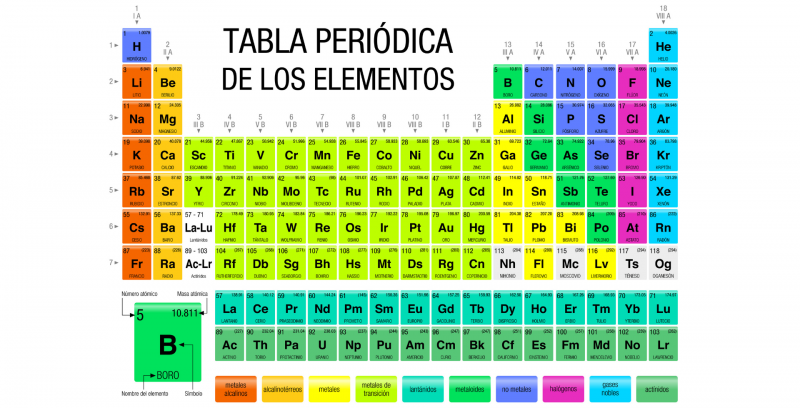
The Periodic Table of the elements is a record of all the chemical elements known to humanity. The elements are arranged in a table according to their atomic number (number of protons), their electronic configuration and their chemical properties.
In this table the elements They are organized in rows and columns that show a certain periodicity: elements that belong to the same column have similar properties. In principle, all known matter in the universe is composed of various combinations of the 118 elements, recorded in the Periodic Table.
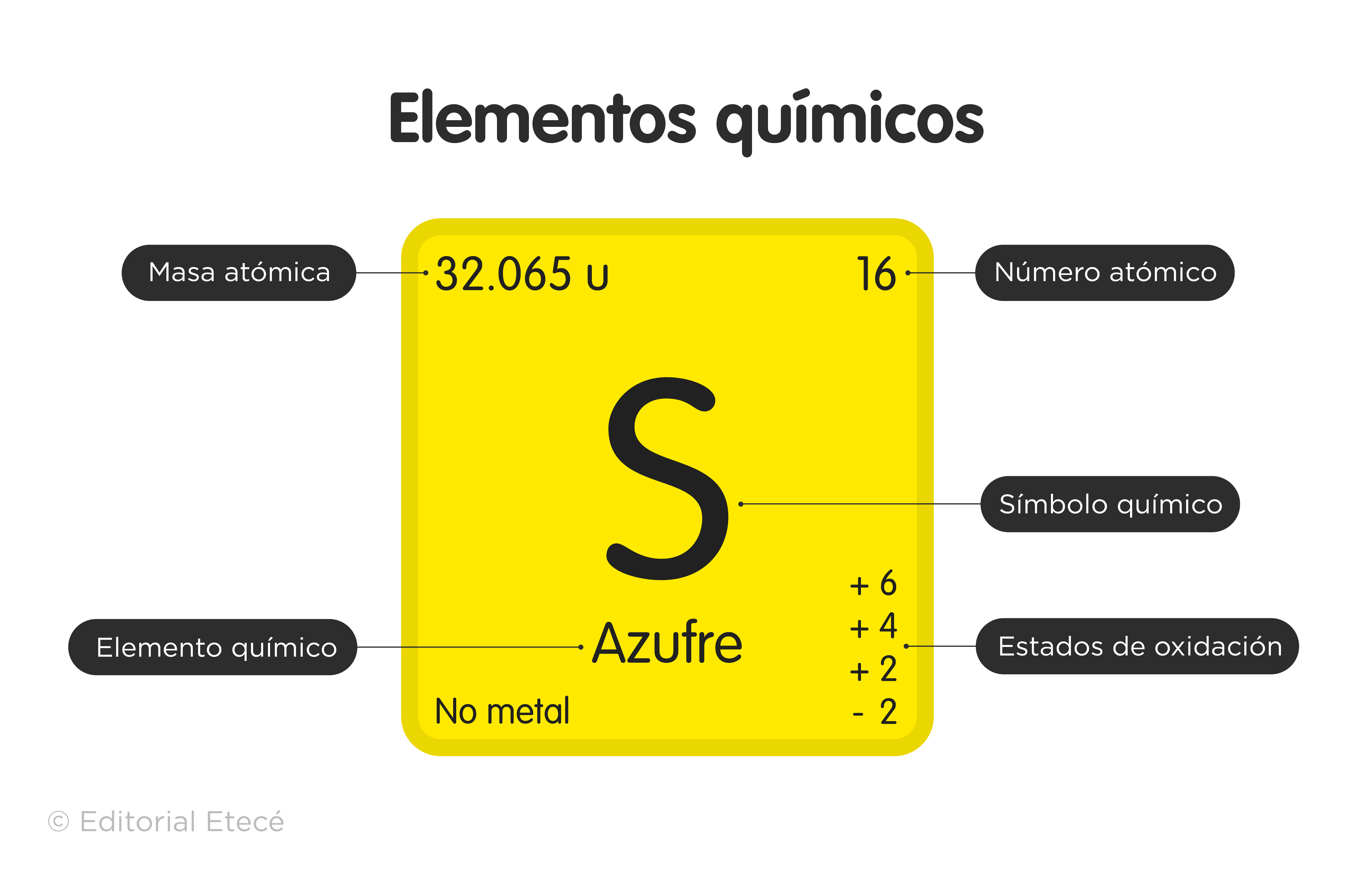
Symbols have been established, called chemical symbols to represent each element of the Periodic Table, which are also identified according to their states of aggregation (solid, liquid or gas) at a temperature of 0 °C and a pressure of 1 atm.
The Periodic Table is a tool fundamental to chemistry, biology and other natural sciences which is updated over the years, as we learn more about the properties of matter and the relationships between elements.
See also: Chemical bond
History of the periodic table
The first version of the Periodic Table was published in 1869 by the Russian chemistry professor Dmitri Mendeleev, and contained 63 of the 118 elements now known in nature and was organized based on their chemical properties. On the other hand, the German chemistry professor Julius Lothar Meyer published an expanded version but based on the physical properties of atoms. Both scholars organized the elements in rows, having the foresight to leave blank spaces where they sensed that there were elements yet to be discovered.
In 1871 Mendeleev published another version of the Periodic Table which grouped the elements according to their common properties in columns numbered from I to VIII according to the oxidation state of the element.
Finally, in 1923 the American chemist Horace Groves Deming published a periodic table with 18 identified columns, which constitutes the version used today.
How is the periodic table organized?
The current periodic table is structured in seven (horizontal) rows called periods and in 18 columns (vertical) called groups either families. The chemical elements are arranged in increasing order of their atomic numbers, that is, the atomic number increases from left to right in the period and from top to bottom in the group.
The eighteen known groups are:
- Group 1 (AI). Alkali metals: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), francium (Fr). Also in this group is hydrogen (H), which is a gas.
- Group 2 (IIA). Alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), radium (Ra).
- Group 3 (IIIB). The scandium (Sc) family, which includes Yttrium (Y) and the rare earths: lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yt), lutetium (Lu). The actinides are also included: actinium (Ac), thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), americium (Am), curium (Cm), berkelium (Bk ), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No) and lawrencium (Lr).
- Group 4 (IVB) The titanium (Ti) family, which includes zirconium (Zr), hafnium (Hf) and rutherfordium (Rf), the latter synthetic and radioactive.
- Group 5 (VB) The vanadium (V) family: niobium (Nb), tantalum (Ta) and dubnium (Db), the latter being synthetic.
- Group 6 (VIB) The chromium (Cr) family: molybdenum (Mb), tungsten (W) and seaborgium (Sg), the latter being synthetic.
- Group 7 (VIIB) The manganese (Mn) family: rhenium (Re), technetium (Tc) and bohrium (Bh), the latter two are synthetic.
- Group 8 (VIIIB) The iron (Fe) family: ruthenium (Ru), osmium (Os) and hassium (Hs), the latter synthetic.
- Group 9 (VIIIB) The cobalt (Co) family: rhodium (Rh), iridium (Ir) and the synthetic meitneiro (Mt).
- Group 10 (VIIIB) The nickel (Ni) family: palladium (Pd), platinum (Pt) and the synthetic darmstadtium (Ds).
- Group 11 (IB) The copper (Cu) family: silver (Ag), gold (Au) and the synthetic roentgenium (Rg).
- Group 12 (IIB) The zinc (Zn) family: cadmium (Cd), mercury (Hg) and the synthetic copernicium (Cn).
- Group 13 (IIIA) The earths: boron (Br), aluminum (Al), gallium (Ga), indium (In), thallium (Tl) and the synthetic nihonium (Nh).
- Group 14 (VAT) Carbonoids: carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb) and the synthetic flevorium (Fl).
- Group 15 (VA) The nitrogenoids: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi) and the synthetic moscovium (Mc).
- Group 16 (VIA) Chalcogens or amphigens: oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po) and the synthetic livermorium (Lv).
- Group 17 (VIIA) Halogens: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At) and the synthetic tenese (Ts).
- Group 18 (VIIIA) The noble gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and the synthetic oganeson (Og).
References
- “The ABC of the Periodic Table” in Chemistry and Society. (PDF)
- “Inorganic Chemistry” by María Dolores de la Llata Loyola. Editorial Progreso (2001) ISBN: 9789706413536.
- “Inorganic Chemistry” by Therald Moeller. New updated version, Editorial Reverté, SA ISBN: 8429173919.