We explain what density is and some characteristics of this property. In addition, other types of density exist.
What is density?
The term “density” comes from the field of physics and chemistry and refers to the relationship that exists between the mass of a substance (or a body) and its volume. This is an intrinsic property of matter, since it does not depend on the amount of substance considered.
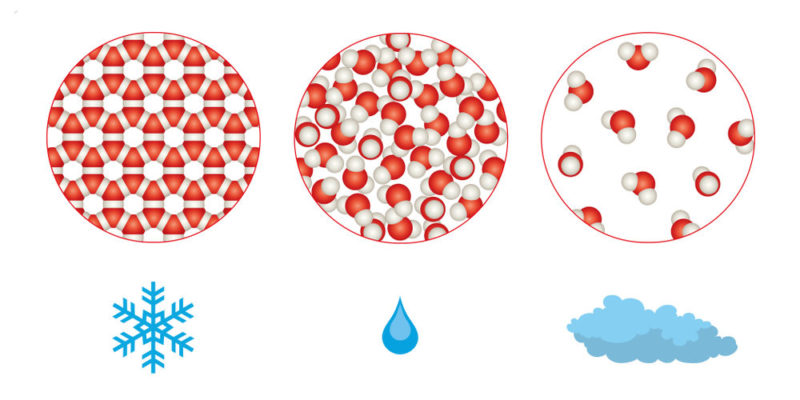
Density, a property that usually It is expressed in kilogram per cubic meter (kg/m3) or gram per cubic centimeter (g/cm3) varies to a greater or lesser extent depending on pressure and temperature, and also with changes of state.
Due to the little cohesion between their particles, gases generally have a lower density than liquids and liquids have a lower density than solids.
The density of matter It is often associated with the story of the Greek philosopher Archimedes who was tasked with determining whether their king's crown had been forged using pure gold or whether it had been made from an alloy with other metals.
During an immersion bath, Archimedes realized that he could calculate the volume of the crown by submerging it in water and measuring the displacement of the liquid, without having to melt or break it, and that by knowing the density of gold (which is a constant) he could then weigh the crown and determine (using the formula) whether it was pure gold or an alloy (the density of the gold would have varied when mixed with other metals).
Although there are exceptions, generally as the temperature increases the density decreases.
Density can be defined in several ways:
- Density or absolute density It is the relationship between the mass and volume of a substance, whether solid, liquid or gaseous. It is represented by the Greek letter rho (𝞺):
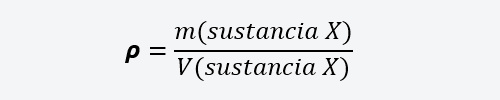
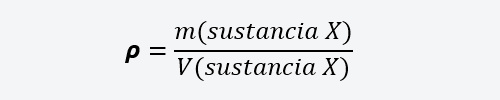
Where m is the mass of a substance and V is its volume. - Relative density It is the relationship between the density of one substance and the density of another substance.
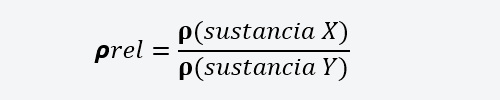
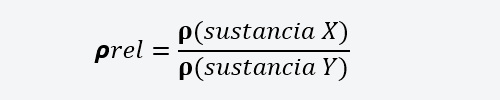
Where 𝞺(substance is the density of the substance x and 𝞺 (substance Y) is the density of the substance ANDwith respect to which the relative density of x. - Apparent density. It is applied to porous materials, which may have air or other substances incorporated between their pores. It is calculated in a similar way to density, but the air mass of the substance that occupies the pores must be added. You must also increase the volume of the substance, incorporating the volume occupied by the substance that occupies the pores.
The density of water is 1 g/cm3 and that of lead is 11.35 g/cm3. In these two examples we can see how density can take very different values in different materials.
See also: Specific weight
Other types of density
- Population density. It is a demographic concept that refers to the number of inhabitants per square kilometer. China and India are examples of countries with very high population density, while the Nordic countries and Oceania have low population density. Areas of high population density are often associated with housing problems, air pollution, insufficient public service infrastructure, among others.
- Optical density. It is a physical parameter that constitutes the absorption of an optical element at a given wavelength per unit distance. This data is used to assess the cell content, quality of smoke generated by different substances, laser power, filters, etc.
- Electric current density It is the relationship that exists between the intensity of electric current that flows through a conductor per unit of time and per unit of cross section.
- Magnetic flux density Also called “magnetic induction,” it is the magnetic flow that causes an electric charge to move for each unit area normal to the direction of the flow.
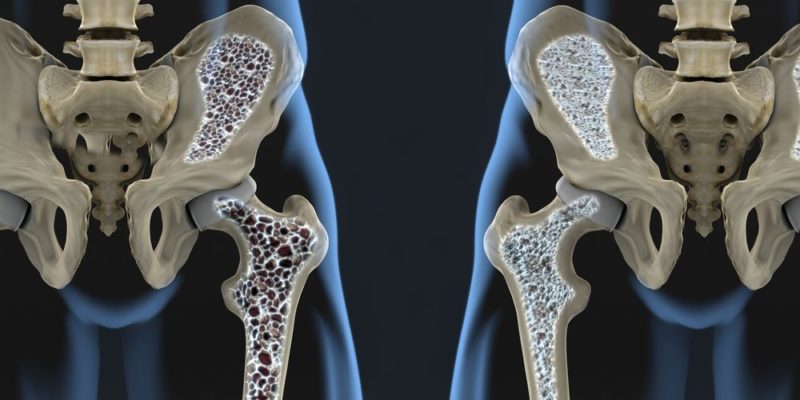
- Bone mineral density In the field of medicine, this measurement refers to the amount of minerals per unit area. It is usually expressed in g/cm2 and is established for certain bones specifically, such as the femur or the lumbar spine. A low bone mineral density can trigger osteoporosis, a disease in which the bones have a very low proportion of minerals (mainly calcium), making them too porous and, therefore, fragile and brittle, thus increasing the risk. of people to suffer fractures.
Examples of the density of some compounds and elements at 20°C
- Magnesium (Mg) 1.738g/cm3
- Calcium (Ca). 1.54g/cm3
- Iron (Fe) 7.874g/cm3
- Molybdenum (Mo) 10.22g/cm3
- Silver (Ag). 10.5g/cm3
- Gold (Au). 19.3g/cm3
- Water (H2O). 1g/cm3
- Oil 0.92g/cm3
- Air 1.225 x 10-3 g/cm3