We explain what water is and what its composition is, what it is for and what its importance is. Also, the types of water, their states and the water cycle.

What is water?
Water is a substance liquid devoid of odor, taste and color which exists in the nature and covers a significant percentage (71%) of the surface of planet Earth. It is composed of two hydrogen atoms and one oxygen atom, so its chemical formula is H₂O.
On our planet, water is mainly contained in the seas and oceans (96.5%), in glaciers and polar caps (1.74%) and in underground deposits (1.72%). The rest of the planet's water (0.04%) is distributed among lakes, soil moisture, atmospheric vapor, wetlands, reservoirs, rivers and streams. In addition, it is a fairly common substance in the Solar system and the universe, although in the form of vapor (its gaseous form) or ice (its solid form).
The planet's water is part of a natural cycle known as the water cycle, in which liquid water evaporates under the action of the sun and rises to the atmosphere in a gaseous state, then condenses in the clouds and falls to the ground as rain. This cycle is vital for the climatic and ecosystem stability of the planet.
- It may help you: Hydrosphere
Characteristics or properties of water
The main characteristics of water are:
- It is colorless, odorless and tasteless.
- It has good electrical conductivity when it has ions dissolved in it and is an electrical insulator in its pure state.
- It is extremely adhesive. Adhesion is the attraction of molecules of the same type or another type. Due to its great adhesion, it can wet objects and bodies.
- Its melting point is 0 °C and its boiling point is 100 ⁰C.
- It has a very stable density, unlike other liquids, its density decreases when it becomes a solid state. That is why ice floats in liquid water.
- It is capable of storing considerable amounts of thermal energy.
- It has a great ability to dissolve a wide variety of substances, making it a powerful solvent.
Most of the planet is made up of salt water, which is found in seas and oceans. Fresh water is found in rivers, streams, lakes, ponds, wetlands and groundwater.
Composition of water
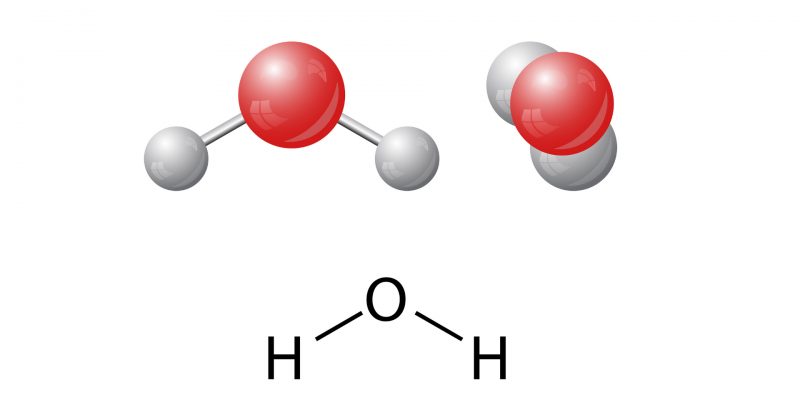
A molecule Water contains only two elements: one oxygen atom and two hydrogen atoms (H2EITHER) joined through a covalent bond. It has a non-linear structure: its two hydrogen atoms are bonded to the oxygen atom and form an angle of 104.5º with each other.
This distribution of its atoms and the high electronegativity value of the oxygen atom generate the formation of an electric dipole that determines the polarity of water.
Water is considered to be the universal solvent, since most substances can dissolve in water. These substances, called “hydrophilic”, are polar. On the other hand, non-polar (apolar) substances, such as oil or gasoline, are called “hydrophobic” and do not dissolve in water.
Additionally, water is a great conductor of electricity and can also retain and store heat.
- See also: Distilled water
States of water

Water, in its natural state, can be found in three fundamental states, each of which is characterized by specific physical properties. These states are: solid, liquid and gas.
- The solid state. Water comes in the form of ice. It is distinguished by its crystalline structure, in which water molecules are arranged in a hexagonal formation. Water reaches this state when it cools below 0°C.
- The liquid state. 97.8% of the planet's water is in liquid form, and is essential for life as we know it. Liquid water has a defined volume but not a fixed shape, so it adapts to the shape of its container. It is found as fresh water in rivers, lakes and groundwater, and as salt water in seas and oceans.
- The gaseous state. At temperatures above 100°C, water boils and turns into water vapor. This state is characterized by the lack of defined volume or shape. Water vapor is a fundamental component in the atmosphere, and has an influence on the climate and all meteorological phenomena.
- States of water
Types of water
The main types of water are:
- Fresh water. It is water with a low percentage of mineral salts. It does not have a sweet flavor, but is called that because its salinity level is low.
- Salt water. It is water with a high percentage of dissolved mineral salts. It is found in seas and oceans.
- Brackish water. It is water with an intermediate percentage of salinity, higher than fresh water but lower than salt water.
- Mineral water. It is water with a high percentage of dissolved minerals. It can be found naturally or artificially mineralized.
- Black water. It is water that is contaminated with animal urine and feces. It is usually found near livestock breeding establishments.
- Gray water. It is water that has already been used for domestic purposes but can be used again for some other domestic use. For example, in many places the water used in the bathroom sink is reused to fill the toilet backpack and, in this way, drinking water is saved.
- Residual water. It is water contaminated by the action of human beings.
- Drinking water. It is water suitable for human consumption, both for drinking and for preparing food or meals. Potable water is little compared to large bodies of non-potable water, such as the sea or rain. It can be found naturally, or it can be made drinkable to remove substances that may be dangerous for consumption.
Water function
Water performs vital functions on the planet, both in aquatic and terrestrial ecosystems. It is a vital means of transporting nutrients and is essential for plant photosynthesis.
In the human body, water is the protagonist of a large number of processes:
- It constitutes the vital medium for most of the body's cells.
- It transports dissolved substances and makes up a large percentage of the blood.
- Facilitates the excretion of substances through urine, feces and sweat.
- Maintains and regulates body temperature.
- Provides electrolytes and minerals essential for the electrical functioning of the body.
Importance of water
The massive presence of liquid water on the planet is one of its main differences with respect to neighboring planets, and It is what allowed the birth and flourishing of life. On the other hand, water, ice, steam and its hydrological cycle maintain climatic stability. In addition, water hydrates the soil and helps make it fertile for plant life and agricultural activity.
For human beings, water is essential for maintaining life. All human societies, from prehistory to the present, are located in regions where they can have access to water. Those civilizations that settled in places where water is a scarce resource have had to develop complex transportation and water supply systems to guarantee their survival.
- See also: Importance of water
The right to water
According to data from the World Health Organization (WHO) and UNICEF, there are approximately 2.2 billion people in the world who lack access to safe and clean water sources. This situation endangers the health and well-being of millions of people in the world who are exposed to contracting diseases from consuming unsafe water.
In 2010, resolution 64/292 of the United Nations recognized the human right to drinking water. This document underlined the importance of safe water consumption for human development and pointed out the urgent need to address the lack of access globally.
Considering water as a fundamental right obliges States to guarantee its access and take measures to prevent water pollution and scarcity. States have the responsibility to implement policies and regulations that ensure universal access to drinking water and sanitation.
Furthermore, the right to water is also linked to other rights, such as the right to health, food and life.
water cycle
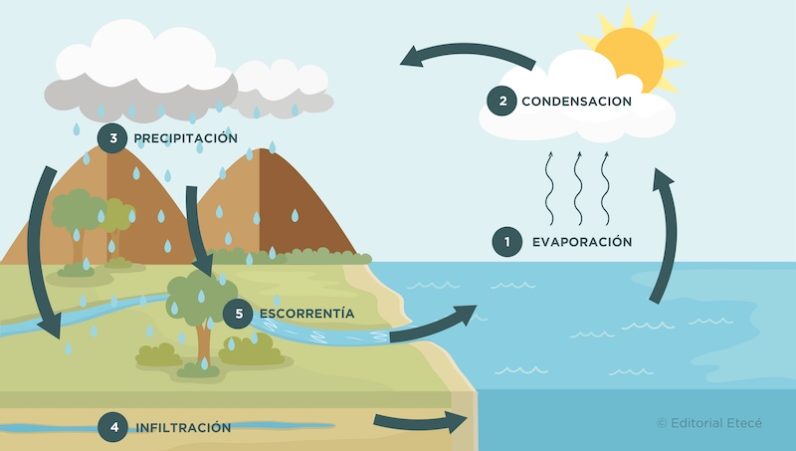
The water cycle is the process of water circulation on planet Earth. During this cycle, water moves and transforms (due to the action of factors such as cold and heat), and goes through the three states of matter: liquid, solid and gaseous.
The water cycle is made up of five stages (evaporation, condensation, precipitation, infiltration, runoff) during which water changes state in a continuous and unlimited cycle.
The water cycle is a vital process because it determines that there is life on the planet and, in addition, it allows ecosystems to be preserved. It is responsible for regulating climates, distributing precipitation, modifying the temperature of the oceans and transporting substances from one place to another.
Thanks to this cycle, water is available to be used by living beings, which obtain it from water courses or from the interior of the earth. In addition, it allows humans to practice most economic and productive activities.
- water cycle
- Sulfur
- Phosphorus
- Nitrogen
References
- UNHCR (2018) The importance of water for life on the planet. United Nations High Commissioner for Refugees. United Nations Organization. https://eacnur.org/
- United Nations Organization (2014) The right to water and sanitation. https://www.un.org/
- Roldán, L (2020) Types of water. https://www.ecologiaverde.com/
- Valdivieso, A. (sf) What is water? https://www.iagua.es/
- Vera, C and Camilioni, A (sf) The water cycle. Ministry of Education, Science and Technology of the Argentine Nation. http://www.bnm.me.gov.ar/